COSMO-RS with multi-species components¶
Sometimes molecules behave in solution in ways that are not accurately captured by the “one-molecule-one-structure” paradigm of standard COSMO-RS theory. The single-structure picture of standard COSMO-RS represents much of the chemical space reasonably well but often fails to accurately describe solutions with more complex behavior. Examples of systems with complex behavior may include:
- Compounds that exist as multiple conformers
- Compounds that may dimerize, trimerize, etc.
- Compounds that can dissociate into multiple species
- Compounds or species that explicitly associate with other molecules (e.g., solvent) in solution
Multi-species COSMO-RS aims to describe the inherent complexities in these types of systems by allowing users to directly input multiple representations for a single component. The ratios of all of these species in solution are calculated and final, “apparent” values are reported for important thermodynamic values with respect to the compounds.
The following tutorial aims to guide the user in setting up and running calculations containing multi-species compounds. This guide will also discuss a few tips and point to a few GUI features that may be useful in understanding the results of the multi-species calculations. Additionally, we will walk the user through a number of practical examples demonstrating the benefits of this approach.
Building multi-species compounds¶
The multi-species compounds input is used to organize information about a compound that can exist in multiple forms. It can be accessed in the COSMO-RS GUI with a single step:
- Select Compounds → Compound with multiple forms
In this window, we can input .coskf files in specific boxes which convey a relationship between a .coskf file and a single molecule of the parent compound or a molecule which must be present for an associated form to occur. .coskf files can be used directly from the AMS sigma profile database or calculated with ADF by following this tutorial. A basic guide for inputting .multiform compounds is given below:
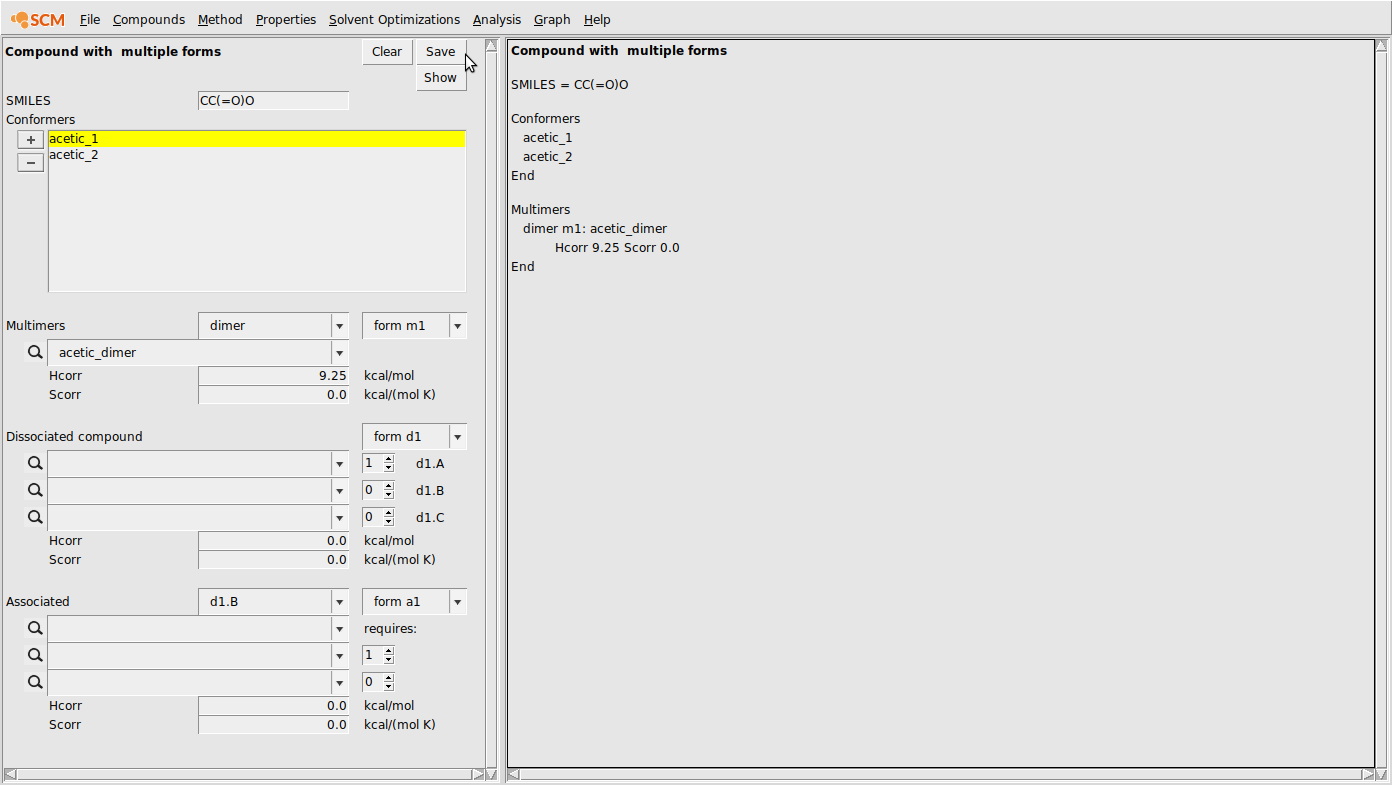
- Select Compounds → Compound with multiple formsInput all conformers under conformersIf there are any dimers, trimers, etc., select the dropdown box next to multimers and choose the appropriate valueEnter the .coskf file and any enthalpy or entropy correctionsIf the compound dissociates, place the appropriate dissociated species in the boxes under the dissociated compound sectionPay attention to the stoichiometry. Note MgCl2dissociates into 1 Mg2+and 2 Cl-ionsIf any structures contain other compounds in the mixture (for association or solvation) place them in the association section.Also, be sure to indicate which additional compounds are required in the following (1 or 2) boxesFinally, click save to save your .multiform file
More details¶
Conformers box¶
In the conformers box, you should input all conformers that have equivalent stoichiometry to the compound of interest. If you want to calculate the chemical potential of a dimer, then all of the compounds on the conformers box should be conformers of the dimer. Conformers may be one of the most common use cases for the multispecies tools as there are many compounds that exist in multiple conformations, and the preferred conformation can be solvent-dependent. Including all relevant conformers in a .multiform file ensures that the most appropriate one is chosen for a the solvent environment.
Multimers box¶
This box is for inputting all multimers of your original compound. For example, any relevant dimers, trimers, etc. should be input here. For clarity, a dimer should have double the amount of each atom, relative to the original compound. To input multiple conformations of a multimer, simply select a new form number in the form drop down menu (this says form m1 by default).
Dissociated box¶
In this box, you can specify forms of a compound that can dissociate. For example, if a compound \(A\) can dissociate in solution to \(B\) and \(C\) , then you would place the .coskf files for \(B\) and \(C\) in the boxes d1.A and d1.B. If \(B\) and \(C\) are both unassociated, then the chemical formulas of \(B\) and \(C\) should add together to produce the chemical formula of \(A\). The Hcorr and Scorr corrections refer to the combination of both dissociated species. Additionally, the numerical box next to the filenames is an indication of how many occurrences of each dissociated form is present. For example, the dissociation \(MgBr_2 \rightleftharpoons Mg^{2+} + 2 Br^-\) would result in the value of 2 in the numerical box next to the \(Br^-\) ion.
Associated box¶
In this box, you can add any complexes that form between 2 or more different compounds/species. For complexes with the same compound, simply use the Multimers box. For example, if Water forms a complex with a dissociated ionic species in the d1.A box, we would simply select d1.A in the first drop down box next to Associated, and then enter the .coskf file for the associated complex as well as the .coskf file for the compound which is associated (in this example, Water). Note that you should also adjust the value in the requires: field if the association is with more than one molecule (e.g., a 2 water association).
Reported chemical potentials¶
When using multiform compounds,the chemical potentials are reported in reference to a single molecule of the base compound. This chemical potential/activity coefficient is the “apparent” chemical potential from the perspective of mixing the multiform compounds. More specifically, in a mixture of \(A\) and \(B\) it can be that many “true” chemical species can occur (\(A\) forms a dimer, \(A\) and \(B\) associate, \(B\) can dissociate, etc.), and each of these “true” species will have an individual chemical potential/activity coefficient. Though these different chemical potentials will be calculated internally, only one chemical potential will be reported per compound, and this is the chemical potential as though that compound only exists in one form. This is a very practical chemical potential value as it relates to the “apparent” mixture and can be used straightforwardly as though the mixture consists only of 2 species.
Note that due to this definition, caution should be taken when defining a base compound. For example, defining NaCl as a base compound with a form that dissociates into Na+ and Cl- is absolutely fine, but all chemical potentials will be reported relative to the combined NaCl species. This can be an issue at infinite dilution: if there are no other sources of Na+ or Cl-, the chemical potential of NaCl goes to \(-\infty\) because the concentration of the combined NaCl species goes to 0 (the entropy increase due to dissociation always prevails at infinite dilution). It may be better in this case to define Na+ and Cl- compounds individually.
Enthalpy and entropy corrections¶
A chemical structure’s energy in solution is calculated as the sum of two energies:
(1) The bond energy, which can be seen in the Compounds → List of Added Compounds. menu; and (2) the solvent-specific corrections calculated by COSMO-RS. While this procedure generally provides acceptable relative energies for different unassociated conformers, it is usually insufficient to calculate relative energies for compounds that associate or dissociate.
It is often necessary to provide corrections for these energies. For example, in the case of acetic acid dimerization, the bond energy alone doesn’t accurately represent the enthalpy and entropy changes involved in dimerization. For this reason, we can add a correction term to the enthalpy (Hcorr) value. Of course, there is a large entropic effect associated with dimerization, but for this simple example, we’ll assume we’re performing a constant-temperature calculation and the Hcorr can effectively represent the entire correction to the free energy. Setting Hcorr to a value of 9.25 for the acetic acid dimer looks like the following:
Example multi-species calculations¶
Conformers and dimers in the acetic acid/hexane system¶
In this example, we’ll investigate the acetic acid/heptane system. In this system, acetic acid prefers to dimerize, making it a good test case to demonstrate the advantages of including multiple forms in a COSMO-RS calculation. We’ll also perform this calculation using only conformers of single acetic acid molecules. Both of these calculations will be compared to the standard COSMO-RS approach using a single structure.
First, we need to generate the .coskf files we need for this calculation. We will consider 3 geometries for acetic acid: 2 unassociated conformers and 1 dimer. These structures are shown below:


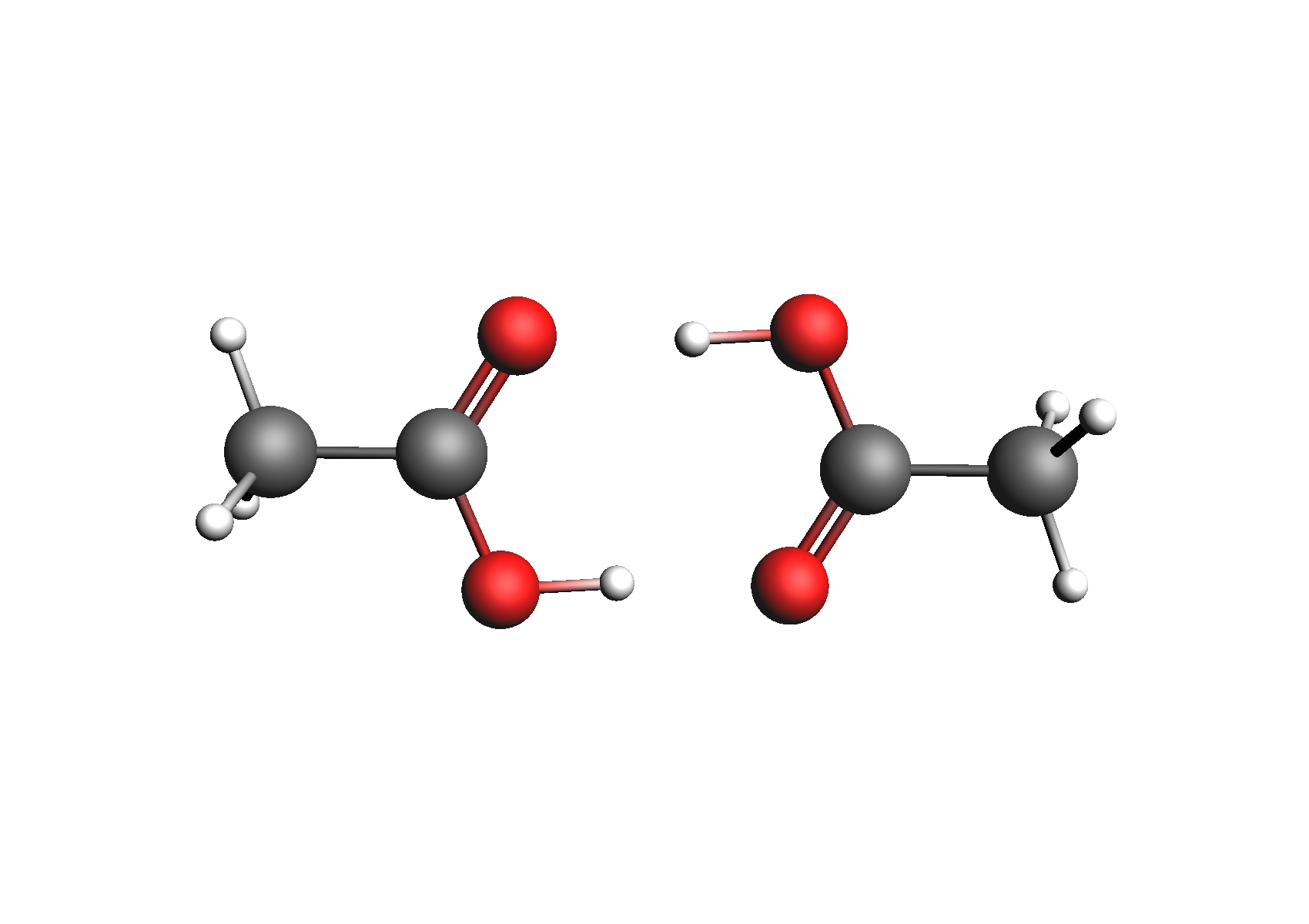
Structures used to represent acetic acid: lowest energy conformer, higher energy conformer, and dimer (clockwise from top left)
First, we will perform a standard COSMO-RS calculation for reference. In this case, we will intentionally choose the higher-energy conformer to illustrate that the results can be significantly influenced by a poor choice of conformer. An additional advantage of using multispecies compounds is that the lowest-energy conformer is preferred automatically in the calculations and the user does not have to exercise so much caution when selecting structures. To do a standard calculation, we perform the following steps:
- Properties → Binary Mixture VLE/LLESelect acetic_2.coskf or compound 1Select Heptane for compound 2Set n to 20Click RunGraph → Y Axis → activities
The result of this calculation is the following:

Next, we will perform the calculation considering 2 conformers for acetic acid. This can be done by creating a .multiform file according to the following:
- Compounds → Compound with multiple formsAdd the acetic_1.coskf under conformersAdd the acetic_2.coskf under conformersHit save in the top of the multiple forms boxSave as acetic_acid_conformers.multiform
Now, we perform the same steps as above in the Binary Mixtre VLE/LLE module, except this time we will use the acetic_acid_conformers.multiform instead of acetic_2.coskf.
This should result in the following image:

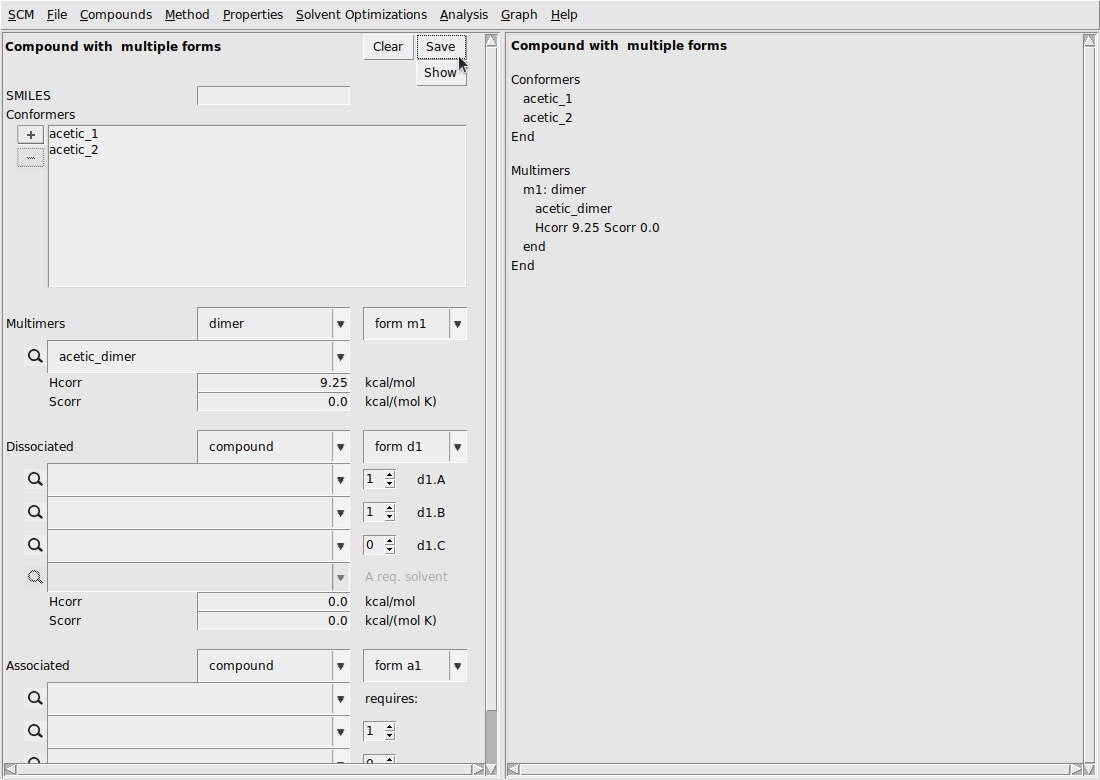
Finally, we build the .multiform file acetic acid with the dimer included. These steps are summarized below:
- Compounds → Compound with multiple formsAdd the acetic acid conformers under conformersAdd the acetic acid dimer under dimerSet the Hcorr of the dimer to 9.25Hit save in the top of the multiple forms boxSave as acetic_acid.multiform
The GUI should look like the following:
Now, we’ll repeat the same steps as above, this time using the acetic_acid.multiform file (including the dimer) in place of the previously used acetic acid file.
The result of this calculation is the following:

Additionally, we can view the relative amounts of the different forms of acetic acid as a function of mole fraction. To view this, do the following:
- Graph → Y Axis → Liquid phase composition 1
This should produce the following:
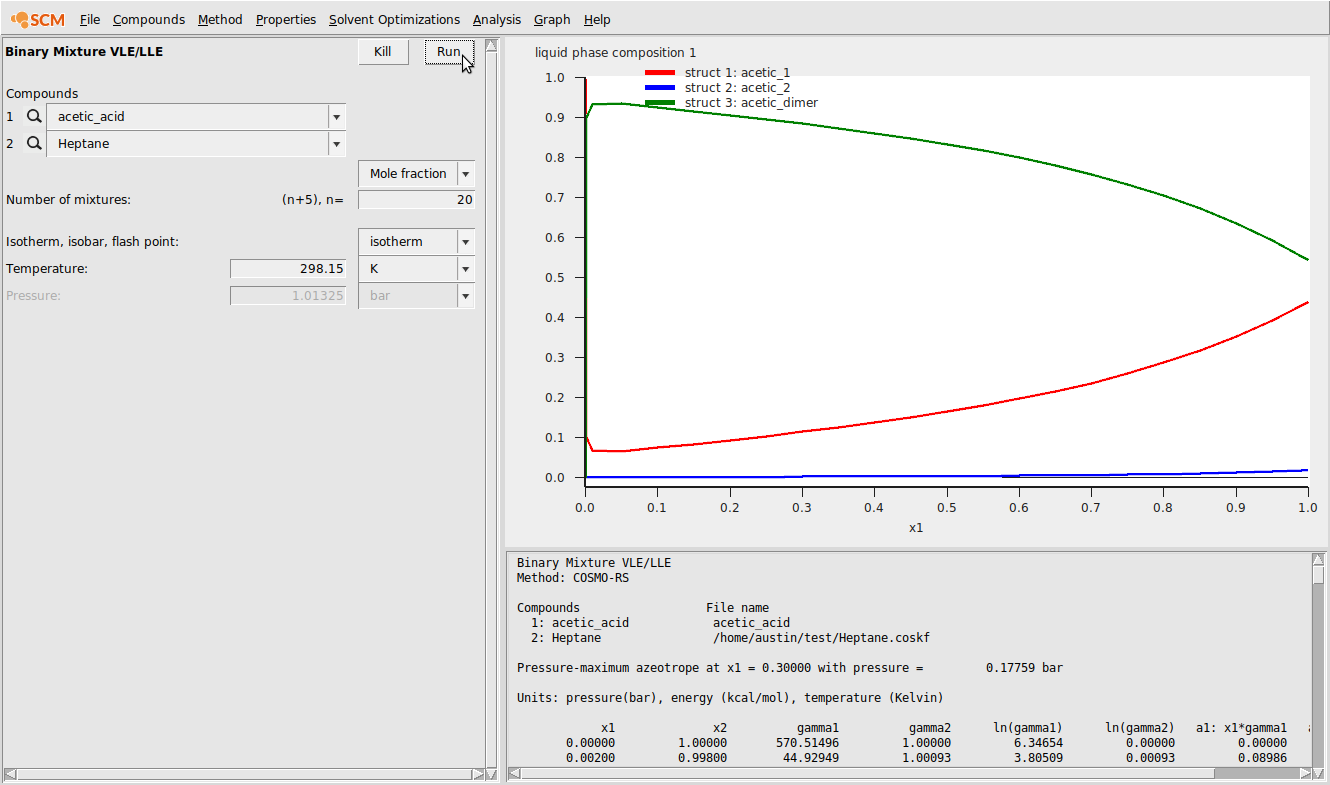
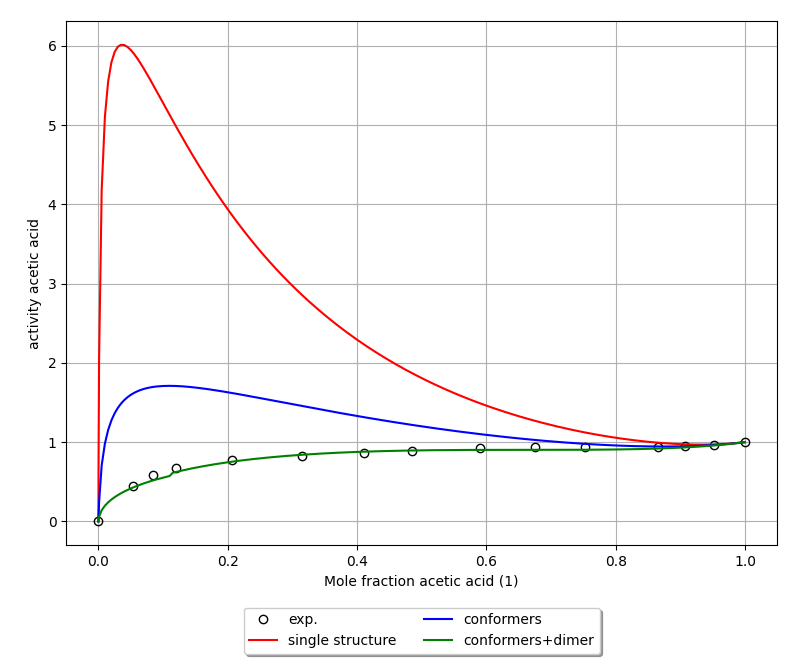
Finally, we can compare the calculated activities to experimental values [1] among 3 scenarios: (1) using a single structure for acetic acid that is also not the lowest energy conformer; (2) using two conformers for acetic acid; and (3) using both conformers and the dimer.
Solvent association in the diethyl ether/chloroform system¶
In this example, we’ll investigate the effect of an association that takes place in the diethyl ether/chloroform system. This effect is observed because the hydrogen atom in chloroform is electron deficient and forms a H-bond with the oxygen in diethyl ether. We begin this example by generating .coskf files for the three relevant structures: diethyl ether, chloroform, and the associated complex. These files can be created by the user by following the steps outlined in the COSMO result files tutorial . Alternatively, the required coskf files can simply be downloaded using the link above (recommended). The structures used in this example are shown below.



Structures used in the diethyl ether chloroform system: diethyl ether, chloroform, and the associated complex (clockwise from top left)
First, we’ll simply consider the system without association. This can be done by performing the following steps:
- Input Chloroform.coskf and Diethyl_ether.coskf to the AMS COSMO-RS GUIProperties → Binary Mixture VLE/LLESelect Diethyl_ether for compound 1Select Chloroform for compound 2Click RunGraph → Y Axis → ln (activity coefficients)
This should result in the following:

Now, we’ll create a .multiform file by adding the associated form to diethyl ether (it can also be added to chloroform). This procedure is as follows:
- Compounds → Compound with multiple formsIn the conformers section, add Diethyl_etherIn the associated section, add the associated Diethyl_ether_chloroformAdd chloroform to the requires box and make sure the requires value is 1Save the multiform file
This should produce the following result:

Now, we’ll use the multiform file in place of the diethyl ether file and repeat the binary mixture calculation.
- Input the multiform diethyl ether/chloroform associated complex to the AMS COSMO-RS GUIProperties → Binary Mixture VLE/LLESelect the multiform file for compound 1Select Chloroform for compound 2Click RunGraph → Y Axis → ln (activity coefficients)
This should result in the following:

Finally, we can compare the two approaches to experiment [2]. We observe that the inclusion of the explicit association greatly improves the agreement with experimental values.

References
| [1] | Lark, Bhajan S., et al. Excess Gibbs energy for binary mixtures containing carboxylic acids. 1. Excess Gibbs energy for acetic acid+ cyclohexane,+ benzene, and+ n-heptane. Journal of Chemical and Engineering Data 29.3 (1984): 277-280. |
| [2] | Becker F.; Kiefer M.; Rhensius P.; Schaefer H.D.: Interpretation von Dampfdruckdiagrammen binärer flüssiger Mischungen mit Hilfe von Gleichgewichtsmodellen. Z.Phys.Chem.(München) 92 (1974) 169-192 |