Chemical Bonding Analysis
The DFT codes ADF and BAND come with various analysis tools offering a detailed insight in the nature of individual chemical bonds and properties like charges, electron densities and potentials. With the integrated GUI these analysis options can be readily accessed and the analysis results are easily visualized in a clear and concise fashion.
Check out our analysis tutorials with examples for EDA, periodic EDA with NOCV, COOP, QTAIM, IQA, Fukui functions, etc. Request a free evaluation to try for yourself.
The energy decomposition analysis (EDA) or extended transition state (ETS) analysis1 in ADF are powerful methods to dissect the interactions that constitute a chemical bond between fragments in a molecule:
∆Ebond = ∆Estrain + ∆Eint
∆Eint = ∆Velstat + ∆EPauli + ∆Eoi + ∆Edisp
The total bonding consists of the interaction energy ∆Eint between the fragments and ∆Estrain, the strain or preparation energy involved in deforming the fragments to the geometries in the pro-molecule.
The interaction energy is further decomposed in terms which are more akin to standard concepts of chemistry. The electrostatic attraction ∆Velstat and Pauli repulsion ∆EPauli are sometimes conveniently summed together into a steric repulsion term.2
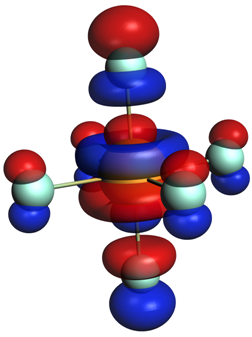
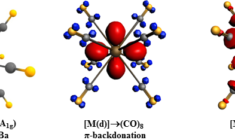
The stabilizing orbital interactions ∆Eoi describe the orbital mixing and charge transfer between the fragments when they form the molecule. In a symmetric molecule, these can be further decomposed into individual irreducible representations of the corresponding point group symmetry. Alternatively, an ETS-NOCV analysis3 decomposes the bonding interactions in the context of natural orbitals for chemical valence (NOCV).
1 T. Ziegler & A. Rauk, Theoret. Chim. Acta 45, 1-10 (1977).
2 F. M. Bickelhaupt & E. J. Baerends, In: Reviews in Computational Chemistry Vol. 15; K. B. Lipkowitz, D. B. Boyd, Eds. (2000)
3 M. Mitoraj & A. Michalak, J Mol Model. 13, 2 (2007)