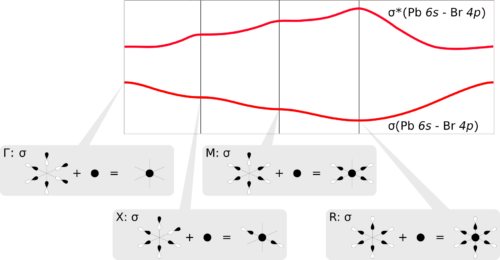
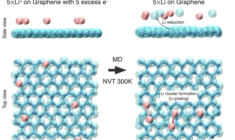
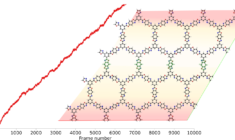
Computational Materials Science: Modeling Materials
Predict and understand material properties through atomistic simulations with the powerful computational chemistry package Amsterdam Modeling Suite (AMS). Speed up research and development of new catalysts, polymers, batteries, and organic electronics through materials modeling. AMS is easy to install, offers molecular (ADF) and periodic density functional theory (BAND, interface to Quantum ESPRESSO and VASP), fast tight-binding (DFTB) and semi-empirical (MOPAC) modules, reactive force fields (ReaxFF), and machine learning potentials.
The integrated graphical user interface as well as through flexible scripting for high-throughput and advanced workflows help you set up and analyze your calculations. The central AMS Driver takes care of advanced potential energy surface exploration tasks (optimizations, scans, molecular dynamics, Monte Carlo, molecule gun) with any program that provides forces and energies. So you can study, at various levels of sophistication, the molecular and bulk properties of systems ranging from a few to a million atoms.