Main Group Elements as Transition Metals: Alkaline Earth Octacarbonyls with 18-electrons
Generally, main group elements prefer obeying the 8-electron rule; while the transition metal elements tend to follow the 18-electron rule. Recently, scientists reported the isolation and spectroscopic identification of the eight-coordinated alkaline earth carbonyl complexes M(CO)8 (M = Ca, Sr, Ba) in a low-temperature matrix, which unexpectedly obey the 18-electron rule.
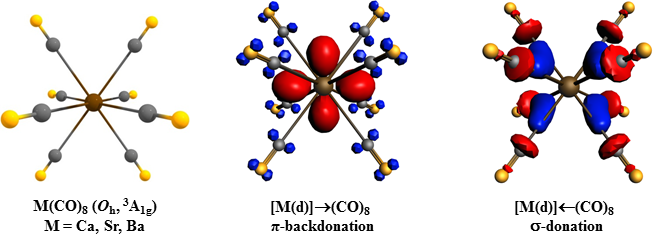
In their recently published Science paper, Xuan Wu et al. used scalar relativistic ADF calculations with EDA-NOCV analysis to show that the metal-CO bonds are mainly due to [M(dπ)]→(CO)8 π backdonation, which explains the strong red-shift of the C-O stretching frequencies. The complexes M(CO)8 exhibit typical features of transition metal complexes obeying the 18-electron rule.
Try it yourself: Request inputfiles
Xuan Wu, Lili Zhao, Jiaye Jin, Sudip Pan, Wei Li, Xiaoyang Jin, Guanjun Wang, Mingfei Zhou, Gernot Frenking, Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals, Science 321, 912-916 (2018)
See also these books:
The Chemical Bond. Fundamental Aspects of Chemical Bonding. G. Frenking and S. Shaik (Eds), Wiley-VCH, Weinheim, 2014.
The Chemical Bond. Chemical Bonding Across the Periodic Table. G. Frenking and S. Shaik (Eds), Wiley-VCH, Weinheim, 2014.
And also check out the chemical bonding analysis page on our website.
Newsletter: tips & tricks, highlights, events
Would you like to keep up to date with the latest developments in the Amsterdam Modeling Suite and the SCM team, learn more about new applications and functionality? Subscribe to our newsletter!
Key conceptsADF bonding analysis ETS-NOCV Relativistic DFT