Analyzing substituent effects
Substitutent effects on aromatic systems are often taught in first year organic chemistry classes from a qualitative standpoint employing resonance structures. A recent paper gives much deeper, quantitative insights in the inner workings of these substituent effects. Appropriately, it made the front cover of the PCCP themed issue: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure.
The substituent effect of the amino and nitro group on the electronic system of benzene has been investigated with quantitative Kohn-Sham molecular orbital theory and the corresponding energy decomposition analyses of the ADF program.
The computations reveal that it is the repulsive interaction between the πHOMO of phenyl radical and the πHOMO of the NH2 radical that is responsible for pushing up the πHOMO of aniline and therefore activating this π orbital of the phenyl ring towards electrophilic substitution.
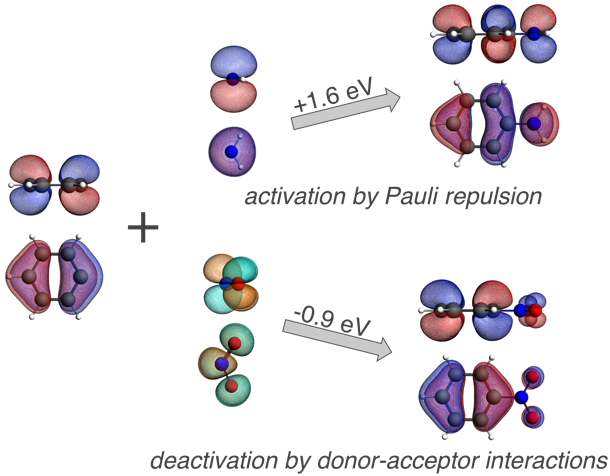
Energy decomposition analysis gives quantitative insight in how substituent (de)activate benzene in electrophilic aromatic substitution reactions.
O. A. Stasyuk, H. Szatylowicz, T. M. Krygowski and C. Fonseca Guerra, How amino and nitro substituents direct electrophilic aromatic substitution in benzene: an explanation with Kohn–Sham molecular orbital theory and Voronoi deformation density analysis, Phys. Chem. Chem. Phys. 18, 11624-11633 (2016)
Key conceptsADF bonding analysis