Characterization of an Atom-Precise Bimetallic Nanocluster
For the first time a bimetallic cluster with a noble metal and a first-row transition metal has been synthesized. The nanocluster was characterized by a joint experimental (ESI-MS) and theoretical (DFT) study.
DFT calculations identified the most stable configuration of the complex nanoparticle, an octahedron with a square-planar Ag4 arrangement and Ni apexes. Fragment analysis revealed that the unusual stability of the nanoparticle originates from charge transfer from the silver atoms to the nickel atoms, combined with the geometrical optimum configuration, maximizing the number of Ag-S bonds. The calculated UV/VIS spectrum of the Ag-Ni nanocluster is in excellent agreement with experiment.
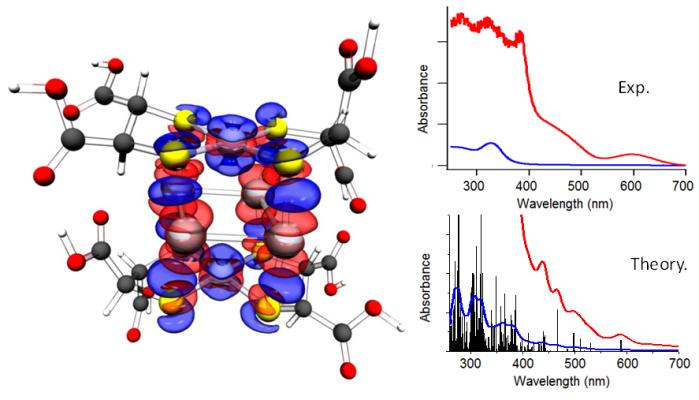
Left: structure and fragment analysis of Ag4Ni2(DMSA)4; red: reduced charge density between the Ag atoms and the Ni(DMSA)2 complexes, blue: enhanced charge density. Right: Experimental and theoretical UV/VIS spectra of the bimetallic cluster.
chemical analysis, TDDFT, ZORA
S. R. Biltek, S. Mandal, A. Sen, A. C. Reber, A. F. Pedicini, S. V. Khanna, Synthesis and Structural Characterization of an Atom-Precise Bimetallic Nanocluster, Ag4Ni2(DMSA)4 , J. Am. Chem. Soc. 135, 26-29 (2013)
Key conceptsADF bonding analysis inorganic chemistry nanoscience Relativistic DFT TDDFT UV/VIS