Chemical analysis of bicyclo(1.1.1) (R2E)3Bi2 complexes
In a recent study researchers Drs. Monakhov and Gourlaouen (University of Strasbourg) investigated fundamental bonding and electronic structure aspects of heteroelemental (R2E)3Bi2 bicyclo(1.1.1)pentane complexes (E = C, Si, Ge, Sn, Pb; R = H) and their ML2-functionalized derivatives (M = Ni, Pd, Pt; L = PH3). These were used as models for experimentally characterized compounds (R’2Ge)3Bi2 and (R’2Ge)3Bi2PtL2 and computationally analyzed in order to understand a broad spectrum of heteronuclear E-Bi, E-M, and Bi-M bonding interactions. This insight could guide synthesis of novel compounds with intriguing bonds.
Insight into the electronic structure of model compounds was obtained with various chemical analysis tools in ADF. Natural bond order (NBO), electron localization function (ELF), and energy decomposition analysis (EDA) indicated that in the non-carbon containing bicyclo(1.1.1) compounds, bonding arises from three carbenoid H2E: units (E = Si, Ge, Sn, Pb) interacting with two Bi(0) atoms. Especially (H2Si)3Bi2 and its (H2Si)3Bi2[ML2] derivatives formed upon the carbenoid-like insertion of ML2 into the Si-Bi bond, show great stability, and are thus attractive synthetic target classes.
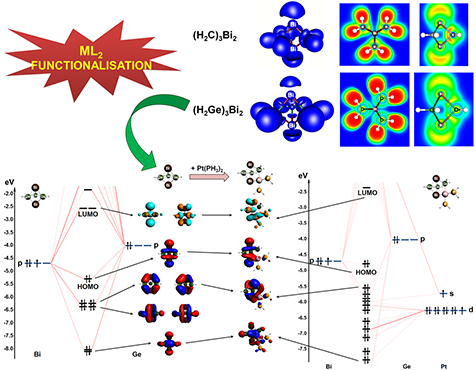
Top: ELF surfaces and contours of the ionically bonded (H2C)3Bi2 and covalently bonded (H2Ge)3Bi2 complexes (ηiso plot is 0.65) show, respectively, the absence and existence of E-Bi basins for the former and the latter and demonstrate the absence of any E-E and Bi-Bi bonding interactions. Bottom: Quantitative MO diagrams for (H2Ge)3Bi2 (left) and its Pt-functionalized derivative (H2Ge)3Bi2[Pt(PH3)2] (right) with most relevant MOs shown (±0.03 isosurface value).
K. Yu. Monakhov and C. Gourlaouen, On the insertion of ML2 (M = Ni, Pd, Pt; L = PH3) into the E-Bi bond (E = C, Si, Ge, Sn, Pb) of a bicyclo[1.1.1]pentane motif: A case for a carbenoid-stabilized Bi(0) species? Organometallics 31, 4415-4428 (2012)
Key conceptsADF bonding analysis inorganic chemistry Relativistic DFT