Growth Mechanism of Small Endohedral Metallofullerenes
In two recent combined experimental and computational studies, the optimal cage for each endohedral metallofullerene within the families Ti@C2n and Ca@C2n has been identified and key aspects of the intriguing growth mechanisms of fullerenes were unravelled. All optimal isomers from C26 to C50 are linked by a simple C2 insertion, with the exception of a few carbon cages that require an additional C2 rearrangement. The large formation abundance observed in mass spectra for Ti@C28 and Ti@C44 on one hand, and Ca@C50 and Ca@C60 on the other, can be explained by the special electronic properties of these cages and their higher relative stabilities with respect to C2 reactivity. Extrusion of C atoms from an already closed fullerene is much more energetically demanding than forming the fullerene by a bottom-up mechanism.
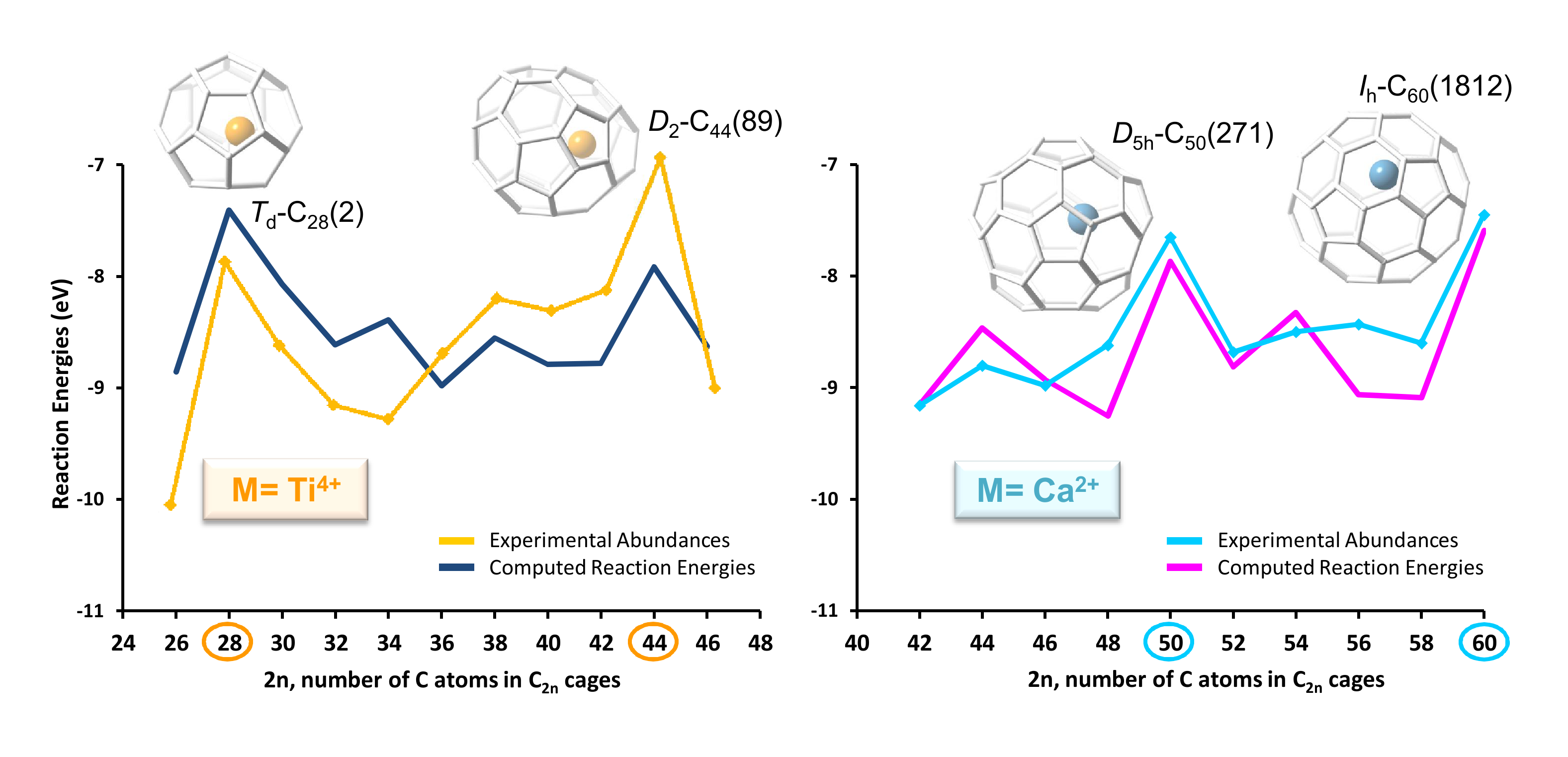
Correlation between observed abundances and computed reaction energies for the next C2 insertion step for Ti@C2n (left) and Ca@C2n (right).
Reactivity, nanomaterials, bonding analysis
M. Mulet-Gas, L. Abella, P. W. Dunk, A. Rodriguez-Fortea, H. W. Kroto, and J. P. Poblet, Small endohedral metallofullerenes: exploration of the structure and growth mechanism in the Ti@C2n (2n = 26–50) family, Chem. Sci., 6, 675-686 (2015)
P. W. Dunk, M. Mulet-Gas, Y. Nakanishi, N. K. Kaiser, A. Rodriguez-Fortea, H. Shinohara, J. P. Poblet, A. G. Marshall, and H. W. Kroto, Bottom-up formation of endohedral mono-metallofullerenes is directed by charge transfer, Nature Comm. 5, 5844 (2014)
Key conceptsADF bonding analysis inorganic chemistry nanoscience Reactivity