Hydrogen bonds in guanine quartets: cooperative effects
The cooperative reinforcement between hydrogen bonds in Guanine quartets (G4) are not caused by resonance-assisted hydrogen bonding (RAHB). Extensive energy decomposition and Kohn-Sham MO and analyses by Fonseca Guerra (VU Amsterdam) et al. reveal that these telomeric structures are stabilized by a new mechanism for hydrogen bond cooperativity. Through donor-acceptor orbital interactions in the s-electron system, hydrogen bonds induce charge separation across the guanine bases in the quartets, stabilizing the system.
The analyses were performed in ADF with dispersion corrected DFT on G4 and Xanthine (X4) quartets in the gas phase, in aqueous solution, and in telomere-like stacks. These findings overthrow the paradigm that the cooperative strengthening is caused by resonance in the p-electron system. The proposed cooperativity mechanism here is argued to apply, beyond the present DNA quartet model systems, also to other hydrogen bonds showing cooperativity effects.
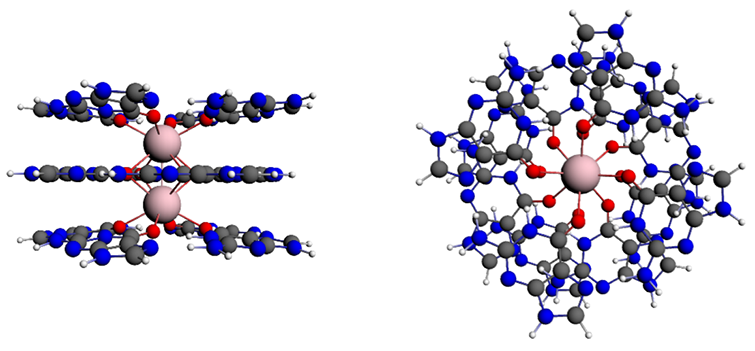
Charge separation in σ framework causes synergistic hydrogen bonding in telomeres.
dispersion-corrected DFT (DFT-D), energy decomposition analysis, solvation (COSMO)
C. Fonseca Guerra, H. Zijlstra, G. Paragi, and F. M. Bickelhaupt, Telomere Structure and Stability: Covalency in Hydrogen Bonds, Not Resonance Assistance, Causes Cooperativity in Guanine Quartets. Chem. Eur. J. 45, 12612 (2011)
Key conceptsADF bonding analysis Dispersion pharma solvation