Microstructure of doped lead halide perovskites from solid-state NMR
Multi-component lead halide perovskites have recently emerged as new promising materials for solar cells and light emitting devices. Essential to their remarkable performance is the notion of doping with inorganic and organic cations such as cesium, rubidium, potassium and guanidinium.
Researchers from EPFL (Lausanne, Switzerland) have used 133Cs, 87Rb and 39K solid-state NMR to probe the local structure of various halide perovskites used in optoelectronics and unambiguously shown that, while cesium is readily incorporated, rubidium and potassium do not incorporate into hybrid organic-inorganic lead halide perovskites.[1-2] ADF allowed them to model structures in which all three cations are embedded inside the perovskite lattice and subsequently calculate their expected 133Cs, 87Rb and 39K NMR shifts. While cesium incorporation yielded shifts consistent with the experiment, the rubidium and potassium case led to shifts far removed from any of the resonances observed experimentally.
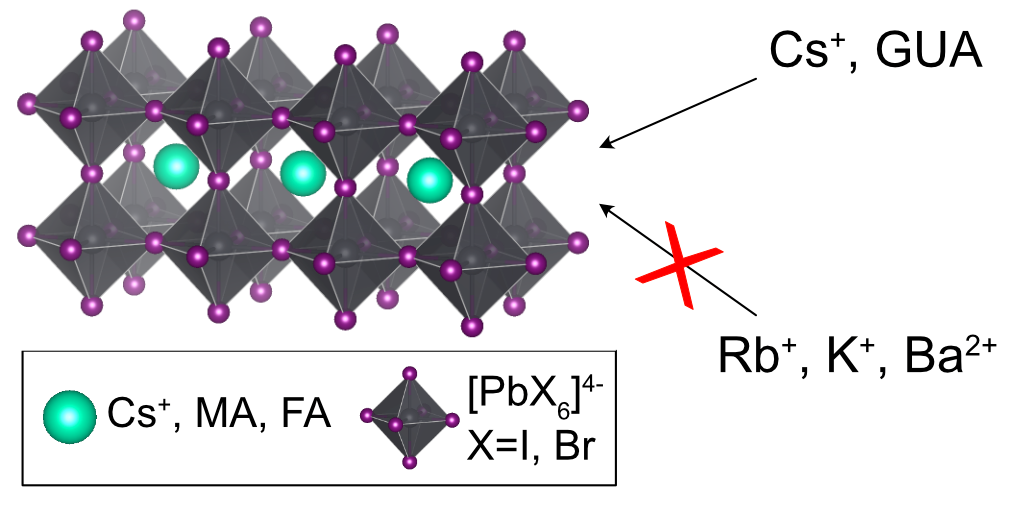
In a similar vein, the group has used 137Ba and 133Cs solid-state NMR experiments combined with ADF shift calculations to show that barium ions are not incorporated into the all-inorganic CsPbI2Br perovskite and cause phase segregation in the material.[3] In another study they employed 14N electric field gradient (EFG) calculations to corroborate the incorporation and dynamics of guanidinium inside methylammonium lead iodide perovskite lattice, thereby solving a long standing problem in the optoelectronic materials community.[4]
NMR calculations in lead halide perovskites are particularly challenging to treat due to the presence of heavy atoms. Chemical shift calculations and EFG were performed at DFT level using the GGA BP86 functional with all-electron TZ2P basis functions including relativistic effects (with spin–orbit coupling) with the ZORA approximation and the Grimme dispersion correction implemented within ADF.
Sample input files for NMR calculations for K and Cs & Ba are available. The bonding analysis in perovskite tutorial may also be of interest.
[1] Kubicki, D. J.; Prochowicz, D.; Hofstetter, A.; Zakeeruddin, S. M.; Grätzel, M.; Emsley, L. Phase Segregation in Cs-, Rb- and K-Doped Mixed-Cation (MA)x(FA)1–xPbI3 Hybrid Perovskites from Solid-State NMR. J. Am. Chem. Soc. 2017, 139 (40), 14173–14180.
[2] Kubicki, D. J.; Prochowicz, D.; Hofstetter, A.; Zakeeruddin, S. M.; Grätzel, M.; Emsley, L. Phase Segregation in Potassium-Doped Lead Halide Perovskites from 39K Solid-State NMR at 21.1 T. J. Am. Chem. Soc. 2018, 140 (23), 7232–7238.
[3] Xiang, W.; Wang, Z.; Kubicki, D. J.; Tress, W.; Luo, J.; Prochowicz, D.; Akin, S.; Emsley, L.; Zhou, J.; Dietler, G.; et al. Europium-Doped CsPbI2Br for Stable and Highly Efficient Inorganic Perovskite Solar Cells. Joule 2019, 3 (1), 205–214.
[4] Kubicki, D. J.; Prochowicz, D.; Hofstetter, A.; Saski, M.; Yadav, P.; Bi, D.; Pellet, N.; Lewiński, J.; Zakeeruddin, S. M.; Grätzel, M.; et al. Formation of Stable Mixed Guanidinium-Methylammonium Phases with Exceptionally Long Carrier Lifetimes for High-Efficiency Lead Iodide-Based Perovskite Photovoltaics. J. Am. Chem. Soc. 2018, 140 (9), 3345–3351.
Key conceptsADF heavy elements NMR optoelectronics perovskite Relativistic DFT solar cells