New organic electrolytes for aqueous redox flow batteries by molecular design
In a recent Science paper, PNNL researchers show that by judiciously designing fluorenone derivatives, the reoxidation of the reduced fluorenol can be achieved reversibly in an aqueous redox flow battery. The combined experimental and computational study is a huge step forward in developing safe organic-based redox flow batteries.
Through tweaking the acidity of the fluorenol proton by adding electron-withdrawing groups such as sulfone while simultaneously improving water solubility by using asymmetric substitution (e.g. sulfone + carboxyl), reversible reduction without external oxidants or catalysts was achieved.
Molecular design of these and other promising organic redox couples can be accelerated by virtual screening of redox potentials and pKa values through ADF + COSMO calculations.
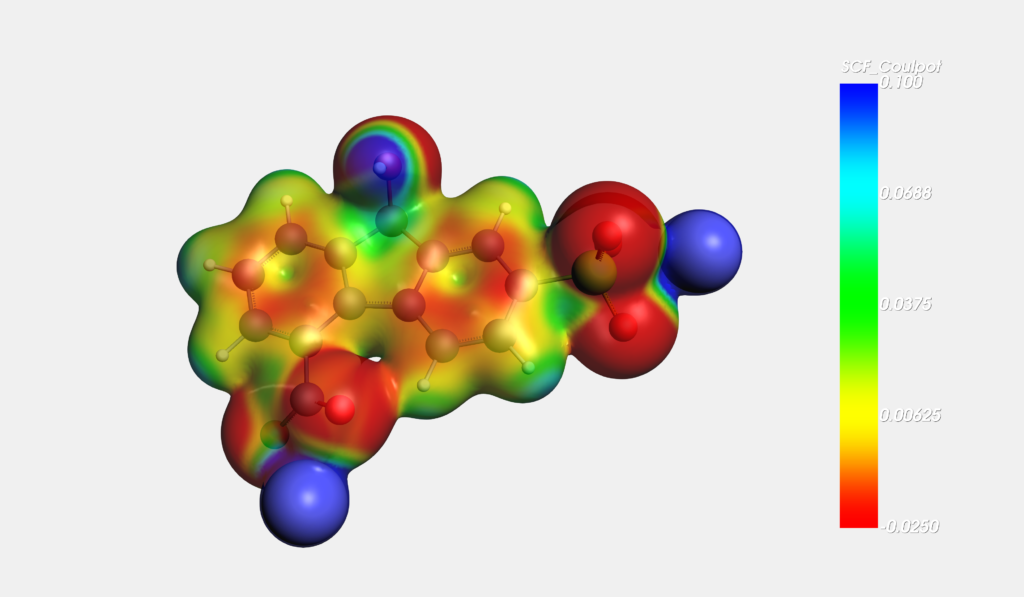
R. Feng at al. Reversible ketone hydrogenation and dehydrogenation for aqueous organic redox flow batteries, Science, 372, 836-840 (2021)
Patent 17/097715: https://uspto.report/patent/app/20210147347