Palladium makes PVC industry greener
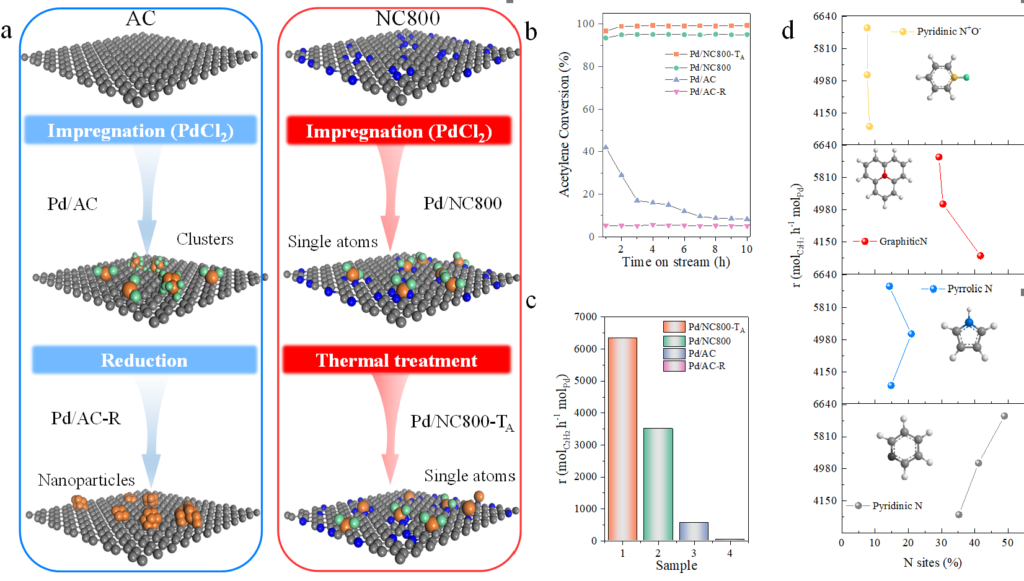
Poly vinyl chloride (PVC) is one of the most widely used polymers with low cost and outstanding performance, which is synthesized through free radical polymerization reaction of vinyl chloride monomer (VCM). Currently, activated carbon (AC) supported mercuric chloride catalyst with typically 10-15 wt.% HgCl2 is still applied in the commercial acetylene hydrochlorination for manufacture VCM. The replacement of the environmentally non-friendly mercuric chloride catalyst with a mercury-free catalyst can effectively solve the bottleneck in terms of green chemistry. To find the best alternative palladium (Pd) catalyst, the team explored the active sites and catalytic mechanism of supported Pd-based catalysts for acetylene hydrochlorination by synthesis and analysis of Pd-based catalysts with distinct nanostructures from nanoparticles, clusters to single-atom site (Fig. 1a). Pd single-atom sites represents higher activity and stability than Pd clusters (Pd/AC-R) or Pd nanoparticles (Fig. 1b-1c). Pyridinic N is shown to contribute to the catalytic activity of Pd single-atom sites (Fig. 1d). The contribution of pyridinic N to the enhancement of catalytic activity is further explored by monitoring synergistic effect, adopting density functional theory (DFT) and conducting kinetic analysis, simultaneously.
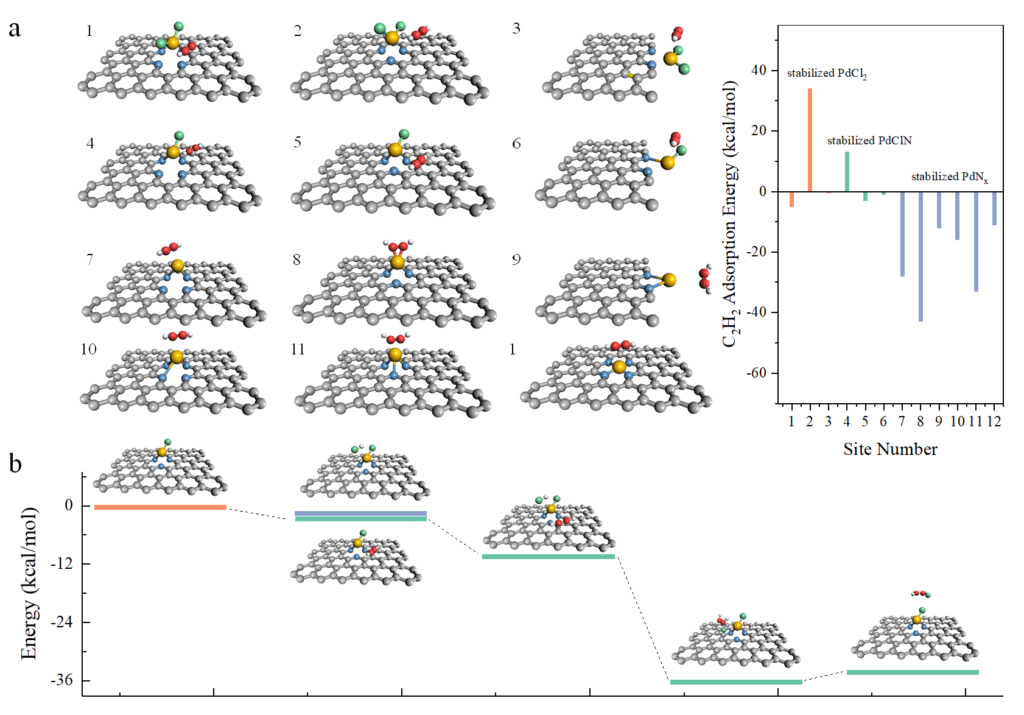
ADF calculations helped to clearly show that the PdN2 species enhances the catalytic efficiency via improving acetylene adsorption (Fig. 2a). In this site, VCM is generated from the reaction of the adsorbed C2H2 at Pd sites, and the adsorbed HCl at pyridinic N sites, following the Langmuir-Hinshelwood (L-H) mechanism with Pd and pyridinic N as dual active sites (Fig. 2b). Kinetic studies reveal that the large contents of pyridinic N configurations on the carrier surface promote HCl enrichment around the Pd sites. This hydrogen chloride enriched strategy provides the possibility to produce vinyl chloride with an equal acetylene/hydrogen chloride feed ratio of 1:1. This is probably the first report about the application of Pd single-atom catalyst in acetylene hydrochlorination and its extension to the realm of targeted applications, which should be very attractive to the chemical industry.
Wang B, Yue Y, Jin C, Lu J, Wang S, Yu L, Guo L, Li R, Hu Z-Ting, Pan Z, Zhao J, Li X, Hydrochlorination of Acetylene on Single-Atom Pd/N-Doped Carbon Catalysts: Importance of Pyridinic-N Synergism, Applied Catalysis B: Environmental (2020).
Key conceptsBAND catalysis Green inorganic chemistry polymers