On the existence of collective interactions in organometallic compounds
In a recent Nature Communications paper, extensive bonding analysis is used to dispute a previous claim on the nature of the Li-C chemical bond. This work involved Prof. Jordi Poater of the IQTCUB institute of the University of Barcelona, Dr. Pascal Vermeeren, Dr. Trevor A. Hamlin, and Prof. F. Matthias Bickelhaupt of the TheoCheM group in the Vrije Universiteit Amsterdam and Prof. Miquel Solà of the DiMoCat group of the Institute of Computational Chemistry and Catalysis of the University of Girona.
The previous paper by Sowlati-Hashjin et al. (Nature Communications 13, 2069 (2022)) concluded that the nature of the Li–C chemical bond in LiCF3 differs significantly from that in LiCPh3 (Ph = phenyl). Whereas the Li–C bond of LiCF3 is classified as a conventional two-center two-electron bond (exchange-correlation interaction collectivity index, ICIXC = 0.910, ICIXC > 0.9 and close to 1), that of LiCPh3 is categorized as a collective bond (ICIXC = 0.393). Sowlati-Hashjin et al. claim that collective bonds take place in systems composed of MAR3 (M = metal; A = C, B or Al; R = substituent) when M forms a stronger bond with the substituents R than with the central atom A. They claim the M–A interaction is either destabilizing or weakly stabilizing, whilst the 1,3-M•••R interactions are strongly stabilizing, but their method does not provide a causal mechanism that would demonstrate the correctness of this interpretation of the ICIXC index.
The recent paper by the theoretical chemists from Spain and the Netherlands proves the opposite, namely, that the Li–CPh3 bond is not reinforced or provided by collective interactions, but that it is weakened by 1,3-M•••R contacts, which reduce the bond overlap. On top of that, there is 1,3-M•••R closed-shell overlap that further reduces the stability through Pauli repulsion. Taken together, these results suggest that there is no need to define the collective interaction as a new type of chemical bond.
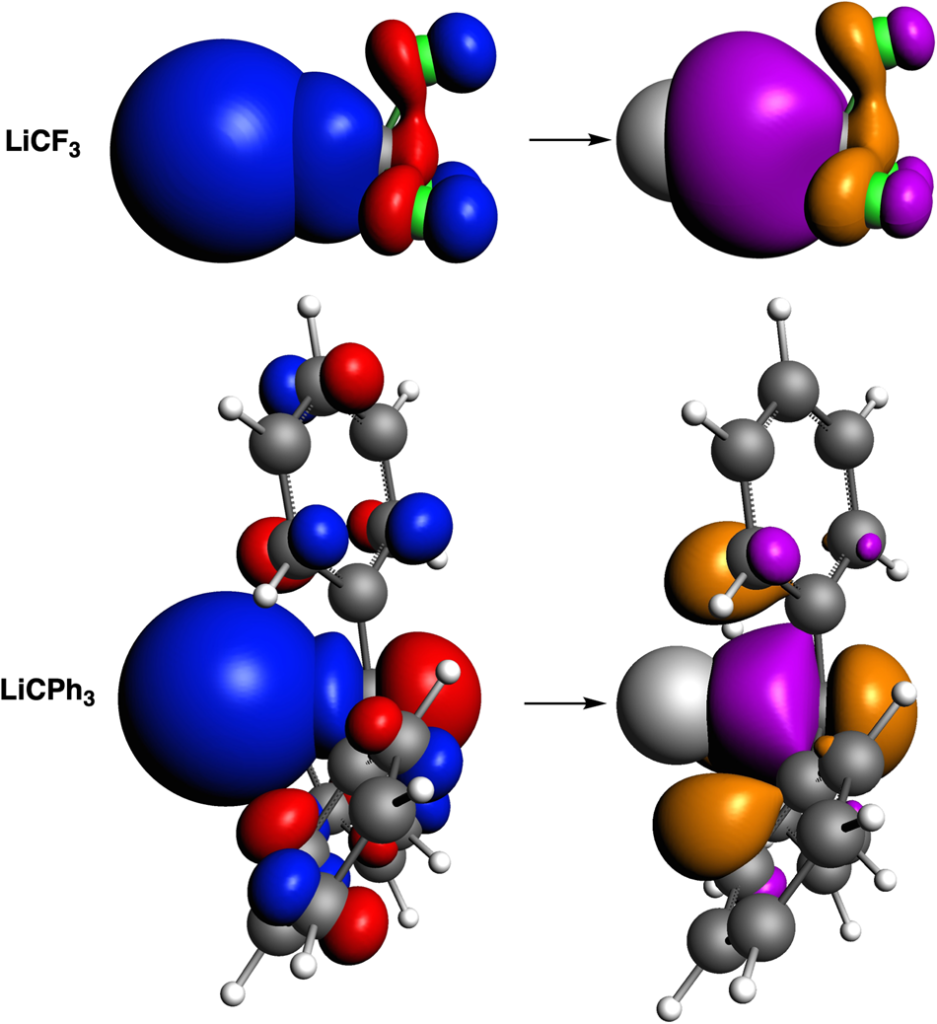
J. Poater, P. Vermeeren, T. A. Hamlin, F. M. Bickelhaupt* and M. Solà*. On the existence of collective interactions reinforcing the metal-ligand bond in organometallic compounds. Nature Commun. 14, 3872 (2023)
Key conceptsADF bonding analysis ETS-NOCV