Rational design of an argon-binding superelectrophilic anion
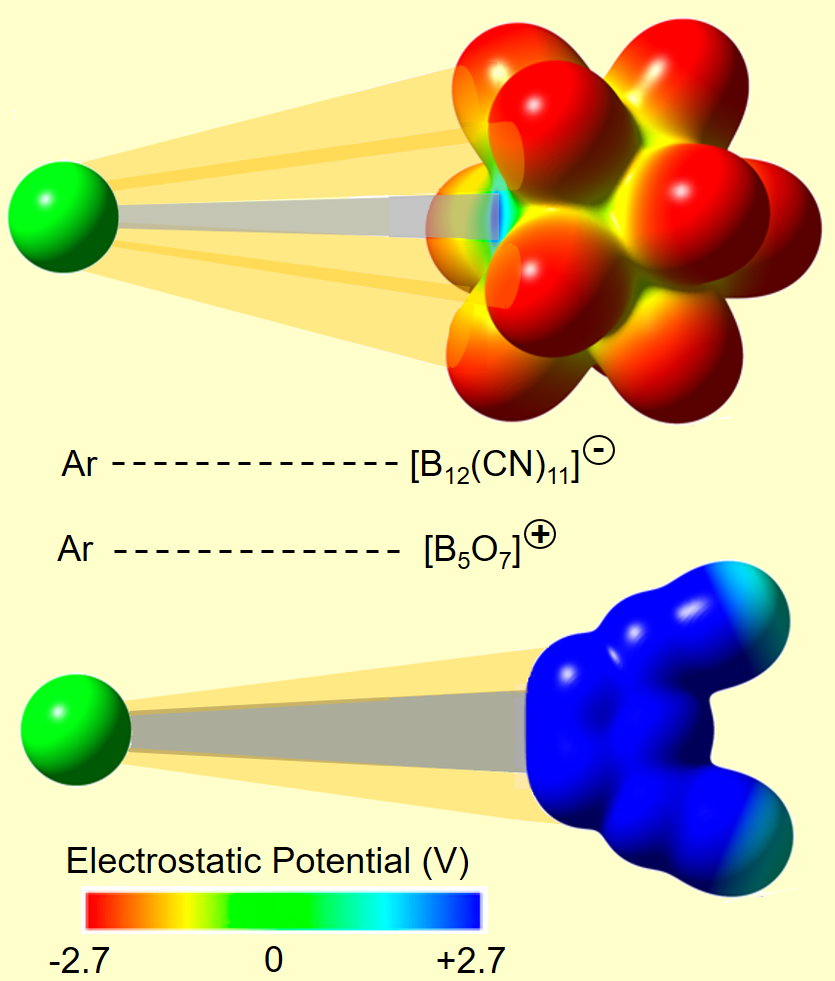
Binding of argon (Ar) at room temperature is assumed to be the privilege of the most electrophilic cations, e.g. [B5O7]+. An international group of researchers from the United States, Germany and South Africa has challenged this intuitive concept by generating the anion [B12(CN)11]– (icosahedral shape with one cleaved vertex) which spontaneously binds to Ar in the gas phase at room temperature. Argon binds to the free boron atom of [B12(CN)11]– which possesses a strongly positive partial charge, although the overall molecular ion charge is negative. Surprisingly, the enthalpy of Ar binding to anionic [B12(CN)11]– was calculated to be very similar to its enthalpy of binding to the oppositely charged [B5O7]+, although this cation exhibits a free boron binding site with significantly higher positive charge than [B12(CN)11]–. In order to understand this phenomenon, the authors chose SCM ADF to perform an Energy Decomposition Analysis (EDA) on both argon compounds. The results revealed a much stronger contribution of electrostatic and dispersion interactions to the attachment energy of Ar to [B12(CN)11]– in comparison to [B5O7]+. Based on this analysis, the authors could conclude that attractive interactions of the bound noble gas atom with the surrounding CN substituents compensates the weaker electrophilicity of the boron binding site in anionic [B12(CN)11]–. Therefore, EDA performed using the ADF software package played a crucial role for understanding the nature of noble gas fixation using these newly discovered electrophilic anions.
Paper
M. Mayer, V. van Lesse, M. Rohdenburg, G.-L. Hou, Z. Yang, R. M. Exner, E. Aprà, V. A. Azov, S. Grabowsky, S. S. Xantheas, K. R. Asmis, X.-B. Wang, C. Jenne, J. Warneke: Rational design of an argon-binding superelectrophilic anion. Proc. Natl. Acad. Sci. U.S.A 2019, 116, 8167–8172.