Resonance-Assisted Halogen Bonds in N-Halo-Guanine Quartets
Due to cooperative effects, the total hydrogen-bond energy in guanine quartets is more stabilizing than four times the bond energy of a single guanine pair (see previous highlight). Based upon the similar bonding mechanism of hydrogen bonds and halogen bonds, researchers from the VU hypothesized that a similar cooperativity effect should be observed when the hydrogen bonds are replaced by halogen bonds in N-halo-guanine quartets.
Density functional computations show that, indeed, halogen bonds possess a similar cooperative effect, which can be even stronger than for hydrogen-bonded quartets. Thorough analyses, using several of the analysis tools available in the ADF suite, reveal that cooperativity is the result of charge transfer interactions that are present in halogen-bonded complexes.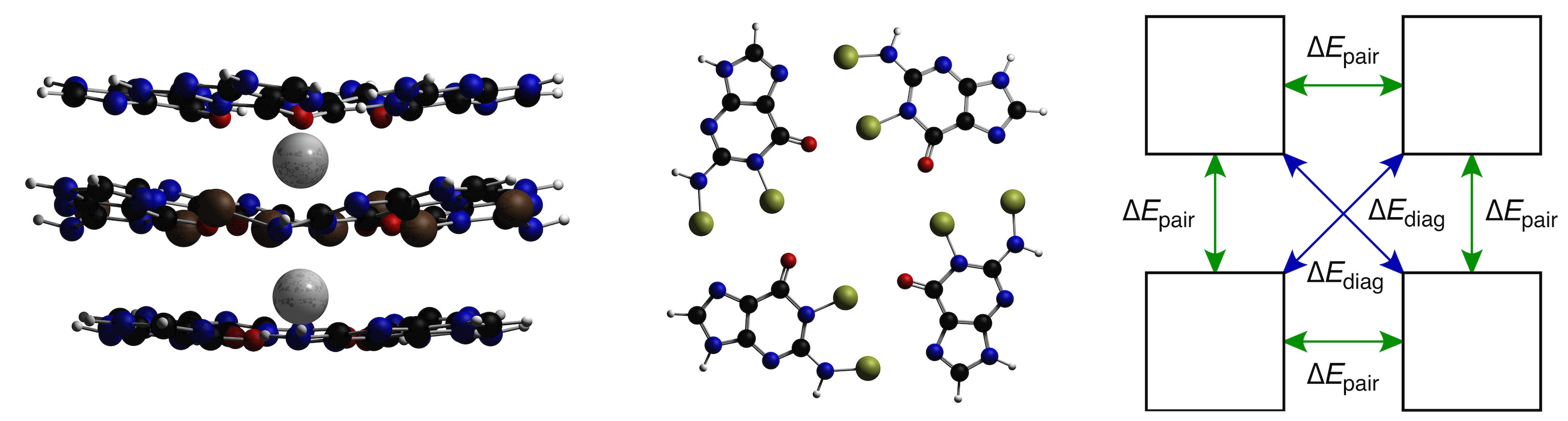
Cooperative bonding interactions in N-halo-Guanine quartets
Energy decomposition, bonding analysis, halogen bonds
L. P. Wolters, N. W. G. Smits, and C. Fonseca Guerra, Covalency in Resonance-Assisted Halogen Bonds Demonstrated with Cooperativity in N-Halo-Guanine Quartets, Phys. Chem. Chem. Phys. 17, 1585-1592 (2015)
Back Cover: Phys. Chem. Chem. Phys. 17, 2275 (2015)