Stable GaX2, InX2 and TlX2 radicals
Group 13 M(II) species are very rare and unstable, yet may well be key electron transfer intermediates. Employing bulky boryl ligands, thermally stable M(boryl)2 complexes (M=Ga,In,Tl) have been isolated and characterized for the first time. DFT calculations by Prof. Kaltsoyannis (UCL) helped to understand the nature of the radicals, and are in agreement with the experimental structural and spectroscopic properties of these stable radicals. The EPR spectra and DFT calculations reveal strong localization of the unpaired electron on the M(II) nucleus, suggesting facile one-electron transfer processes at the metal center of M(II) complexes.
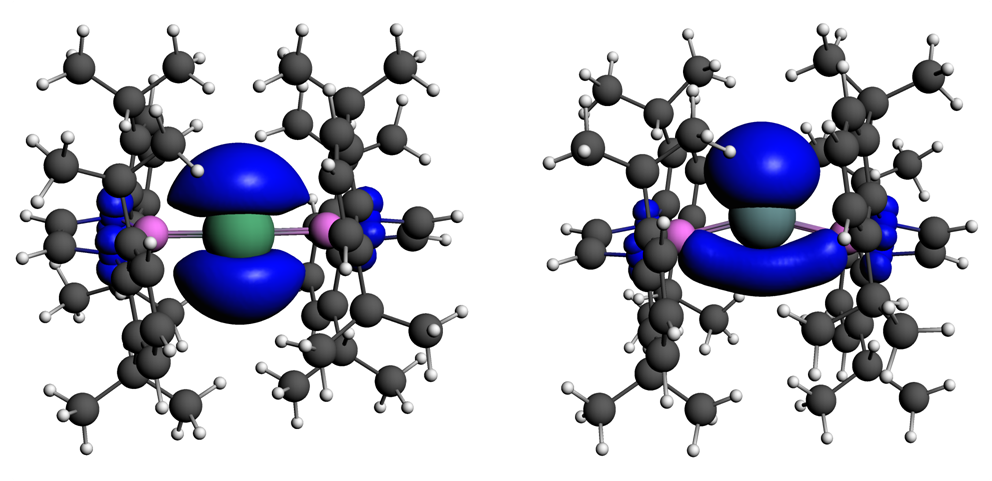
Spin densities for M[B(NDippCH)2]2, M=Tl (left) and M=In (right).
The geometry at the metal center in M[B(NDippCH)2]2 is not the same for all three metals. When M=Tl, the B-M-B angle is almost linear, while for Ga and In it is more bent, although the unpaired electron is still strongly localized on the metal. The bending at the radical center increases in the order M=Tl<Ga<In, which is rationalized in terms of the decreasing energy gap between the metal’s valence s and p orbitals, and hence greater tendency to hybridize.
A. V. Protchenko, D. Dange, J. R. Harmer, C. Y. Tang, A. D. Schwarz, M. J. Kelly, N. Phillips, R. Tirfoin, K. H. Birjkumar, C. Jones, N. Kaltsoyannis, P. Mountford, and S. Aldridge, Stable GaX2, InX2 and TlX2 radicals, Nature Chem., 6, 315-319 (2014)
Key conceptsADF bonding analysis EPR inorganic chemistry Relativistic DFT spectroscopy