Strong AT hydrogen bonds: sp2 enhances electrostatics & covalency, no resonance-assistence
The recent cover story of Chemistry Open pursues the origin of the strong hydrogen bonds in Watson-Crick base pairs. While the commonly held theory of resonance-assisted hydrogen bonding underlying this hydrogen bond strengthening has been contested previously, this study scrutinizes the hydrogen bonds in AT base pair by also studying analogues with a smaller π-framework, and even saturated (sp3) mimics.
Using DFT-D3(BJ) calculations and energy decomposition analysis on the different AT-analogue pairs, it has been established that the π electrons do not enhance hydrogen bonding via resonance-assistence. Instead the π-framework only plays a role in terms of creating a stronger electrostatic interactions and better donor-acceptor overlap, increasing hydrogen bond covalency. Consequently, the sp3-mimics have hydrogen bonds that are half as strong as the hydrogen bonds in the AT base pair and its analogus with sp2-hybridized donor and acceptor centers.
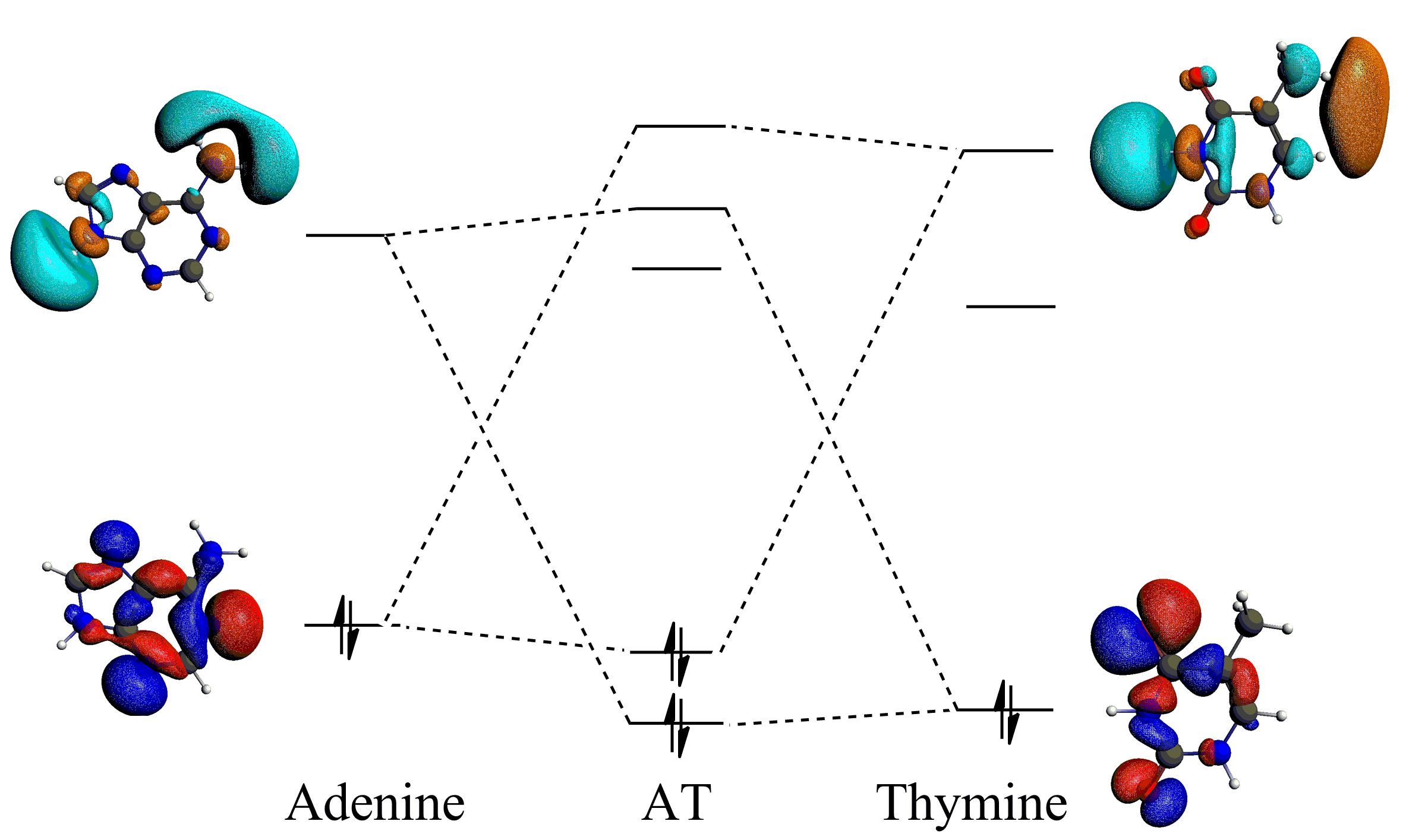
Donor-accpetor interactions for the hydrogen bonds in the AT base pair, enhanced by the sp2 hybridization
See also: energy decomposition analysis
Energy decomposition analysis, charge analysis, hydrogen bonds
L. Guillaumes, S. Simon, and C. Fonseca Guerra, The Role of Aromaticity, Hybridization, Electrostatics, and Covalency in Resonance-Assisted Hydrogen Bonds of Adenine–Thymine (AT) Base Pairs and Their Mimics, Chemistry Open, 4, 318-327 (2015)
Key conceptsADF bonding analysis pharma