Actinide-Lanthanide bonding unraveled
Metal-metal bonding is a classic research topic in chemical bonding studies and has been applied as a tool for developing molecular magnets as well as for addressing challenges in biology, energy, and catalysis. In particular, di-metallofullerenes (di-EMFs), with only two metal atoms trapped inside the carbon cages, i.e. M2@C2n, provide a unique platform to study these bonding interactions.
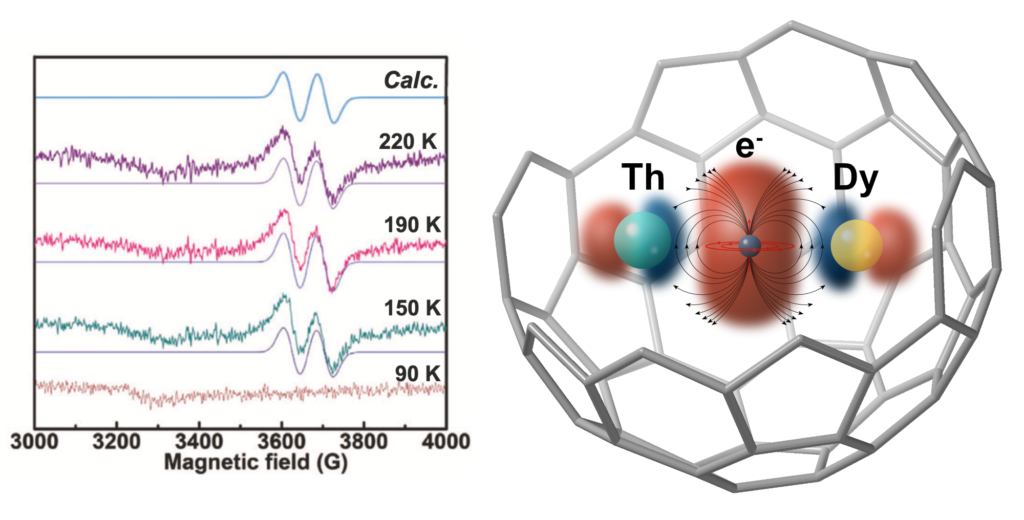
In this article, we report a series of mixed-valence di-metallofullerenes, ThDy@C2n (2n = 72, 76, 78, and 80) and ThY@C2n (2n = 72 and 78), which feature single electron actinide-lanthanide metal-metal bonds, characterized by structural, spectroscopic and computational methods.
DFT and CASSCF calculations identify the presence of a single-electron bonding interaction between Th and Y or Dy, due to a significant overlap between hybrid spd orbitals of the two metals, resulting in a formal oxidation state of +3.5 for Th and +0.5 for Y.
This new wide family of endofullerenes provides experimental proof of the direct metal-metal lanthanide-actinide bond, which will contribute to a better understanding of bonding between f-block elements. Computational studies predict that the coupling of a single electron in the sigma-bonding orbital with 4fn and 5fn on different lanthanides and actinides may give rise to outstanding magnetic properties, which may help in the design and synthesis of next generation molecular magnets.
Y. Yan et al. Actinide-lanthanide single electron metal-metal bond formed in mixed-valence di-metallofullerenes, Nature Comm. 14, 6637 (2023)
Key conceptsADF bonding analysis EPR Relativistic DFT spectroscopy