Boron Nitride Nucleation through Chemical Vapor Depostion
Researchers from the University of Newcastle, Australia have used non-equilibrium ReaxFF molecular dynamics simulations to provide the first glimpse showing how boron nitride (BN) nanostructures form during boron oxide chemical vapor deposition (BOCVD) at the atomic level. These simulations have resulted in a revision of the established BN formation mechanism derived from previous experiments, in which the formation of H2 and H2O vapors play key roles via independent and competing chemical pathways. Whereas H2O formation promotes BN bond formation and the condensation of BN rings, H2 formation instead promotes the self-assembly of boron oxide BxOx chains to form catalytic B nanoparticles. BN initially forms as a highly defective, almost amorphous material, before “healing” into more well-defined network structures. Such processes occur over nanosecond timescales (and longer), making ReaxFF the ideal method for their investigation.
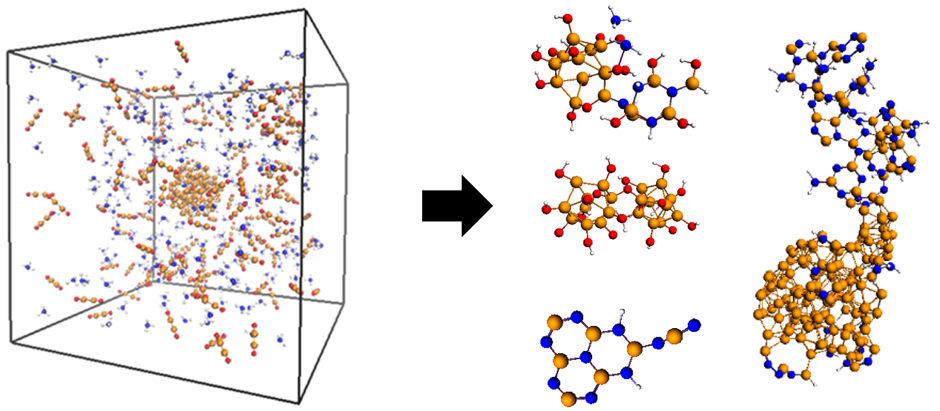
Try for yourself: geometries for B2O2, B2O2 + NH3, and NH3 + BxOy with a higher B/O ratio
B. D. McLean, G. B. Webber, and A. J. Page, Boron Nitride Nucleation Mechanism during Chemical Vapour Deposition, J. Phys. Chem. C 122, 24341 (2018).
Key conceptsCVD materials science nanoscience Reactivity ReaxFF