EDA-NOCV Bonding Analysis of Tin(II) Carbene Complexes
In a recent experimental and computational study a group of researchers from the University of Saarbrücken, Germany, investigated the structure and chemical bonding of tin(II) halide and hal-sandwich complexes with cyclic (alkyl)(amino)carbenes (cAACs). Compared to the well-known N-heterocyclic carbenes (NHCs), cAACs can act as stronger σ-donor and π-acceptors enabling them to stabilize otherwise unstable species of main group compounds.
To unravel the physical factors involved in the coordination of cAACs with different tin species, their bonding interactions were analyzed with EDA-NOCV. The nature of coordination is primarily electrostatic with an important covalent character. The orbital term is dominated by the donation of carbene electron lone pair into the formally empty Sn p-orbital. The strength of this donation is the key to stabilize tin(II) compounds.
Try for yourself: download input files
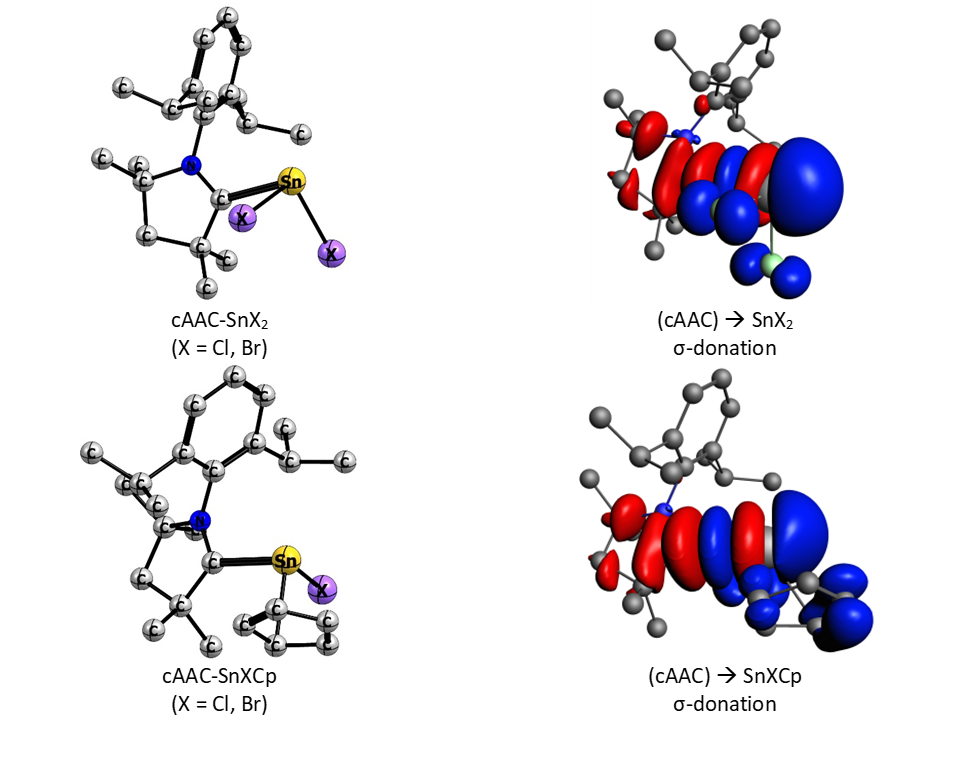
Müller, C.; Andrada, D.M.; Bischoff, I.-A.; Zimmer, M.; Huch, V.; Steinbrück, N.; Schäfer, A. Synthesis, Structure, and Bonding Analysis of Tin(II) Dihalide and Cyclopentadienyltin(ii) Halide (Alkyl)(amino)carbene Complexes. Organometallics 2019.
Key conceptsADF bonding analysis inorganic chemistry