Fast Intrinsic Emission Quenching in Cs4PbBr6 Nanocrystals
Cs4PbBr6 belongs to the family of 0D metal halides, with separated and isolated [PbBr6]4- octahedra. These systems, in both nanocrystal and bulk forms, have been reported as being either non-emissive or green-emissive at room temperature in different and conflicting reports, with no clear agreement on the origin of the green emission or on the quenching mechanism thereof. To disentangle this riddle, researchers from the Nanochemistry Deparment at the Italian Institute of Technology (IIT) evaluated the electronic structure of the isolated lead-halide octahedra moieties that constitute the material and then performed ab initio molecular dynamics of 2x2x2 supercells of the Cs4PbBr6 solid-state material at different temperatures.
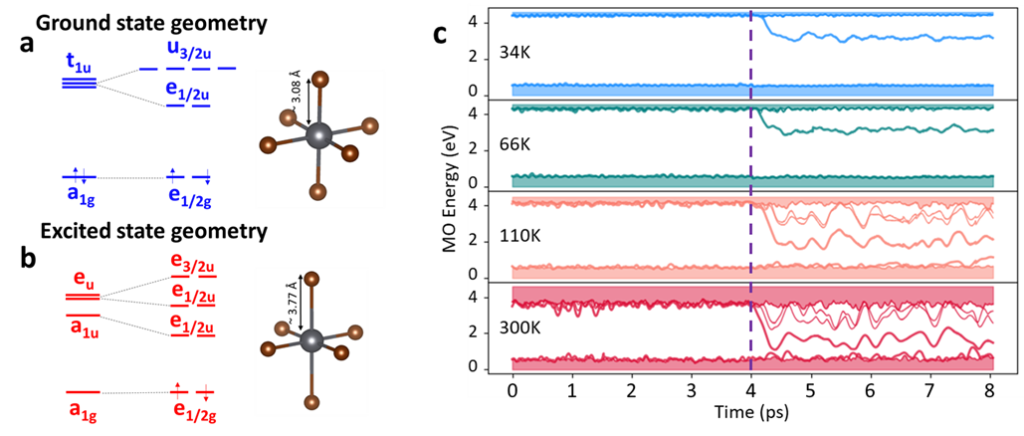
The study of the single octahedron properties, carried out at the DFT(TDDFT)/PBE/DZP level of theory, made it possible to assess the differences between the ground and the lowest excited state geometries and the impact of spin-orbit coupling (SOC) on the energetic levels and the electronic transitions. In particular, geometry relaxation of the excited state produces mainly an axial elongation of the Pb-Br bonds inside the octahedra, with the SOC contribution causing a mixing of the triplet and singlet character of the lowest excited states, rendering spin-forbidden transitions optically allowed, in agreement with photoluminescence excitation and emission spectra.
Molecular dynamics simulations on the full Cs4PbBr6 showed that the coupling of the electron to the phonon mode responsible for Pb-Br elongation causes the quenching of photoluminescence, which therefore appears exclusively at low temperature, in agreement with experimental data.
Inclusion of a CsBr-vacancy defect, considered as one of the main causes of the green emission, resulted again into a quenching of the luminescence, thus pointing to interconnected octahedra as the only possible defect capable of giving rise to green emission.
In conclusion, in the pure material, room temperature emission is suppressed as a consequence of thermal quenching, thus the observed green emission can be only the result of structural defects that cause interconnections between PbBr6 octahedra rather than vacancies or other point defects.
U. Petralanda, G. Biffi, S. C. Boehme, D. Baranov, R. Krahne, L. Manna*, and I. Infante, Fast Intrinsic Emission Quenching in Cs4PbBr6 Nanocrystals, Nano Lett. 21, 8619–8626 (2021)
Key conceptsADF nanoscience Relativistic DFT