The First Dodecavalent Molybdenum Complex studied with ADF
Recently Prof. Gernot Frenking’s group in Marburg, Germany was involved in a joint experimental – theoretical paper that reported the synthesis and characterization of a unique molybdenum – zinc complex in which the zinc atoms coordinate to the central molybdenum in a nearly icosahedral cage. This twelve coordinate structure of zinc atoms about a molybdenum was confirmed in the X-Ray structure depicted below. Their work, published in the October 7, 2008 issue of Angewandte Chemie was also highlighted in the November 10, 2008 issue of C&E News.
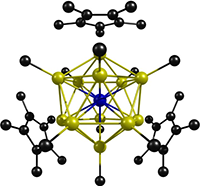
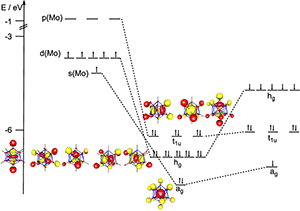
Left: X-Ray Structure of [MoZn12Me9Cp*3]. Right: MO Diagram for [Mo(ZnH)12]
Coordination complexes with up to nine monodentate ligands have been known for over a century, and also well known are metal – metal clusters, in which one type of metal is entrapped in a shell of surrounding atoms (usually more than nine of them) of another type, with the later metals being electronically bound to one another. One aspect that makes this molecule unique, as revealed by density functional theory calculations with ADF, is its electronic structure. Based in part upon these calculations, the average bond order between the center atom and its ligands was determined to be 1/2, with sd5 hybrid orbitals on the molybdenum center participating in the covalent bonding with surrounding zinc atoms. Furthermore, the authors determined that an additional six electrons were delocalized amongst the icosahedron formed by the zinc atoms, resulting in weak bonds between them as well. As such, this molybdenum zinc complex appears to have similarities with traditional metal – ligand complexes, with their covalent bonding primarily from the metal to its surrounding ligands, as well as being similar to endohedral metal – metal clusters, characterized by bonding amongst the metal atoms surrounding another.
ADF’s Energy Decomposition Analysis and Fragment Orbitals were used in this study to obtain a detailed understanding of the electronic structure and bonding of this molecule. According to Prof. Frenking, “the ADF calculations have been crucial for understanding the bonding situation in the molecules because they make it possible to quantitatively estimate the strength of the contributions which come from the s, p and d valence orbitals of the central metal atom.”
T. Cadenbach, T. Bollermann, C. Gemel, I. Fernandez, M. von Hopffgarten, G. Frenking and R. A. Fischer, Twelve One-Electron Ligands Coordinating One Metal Center: Structure and Bonding of [Mo(ZnCH3)9(ZnCp*)3]. Angewandte Chemie International Edition, 47 (47), 9150 (2008).
Key conceptsADF bonding analysis