Gas Solubility in Ionic Liquids
In a recent Chemical Reviews paper researchers from the Beijing University of Chemical Technology have reviewed various models for predicting solubility of gases in ionic liquids (ILs), with a focus on CO2. The predictions of activity-coefficient based methods and equation of state models were compared to experimental data. The group-based method UNIFAC shows better agreement with experiment than COSMO-RS, where parameters exist. However, UNIFAC is not easily extended to gases and ILs outside the parametrization scope. On the other hand, the quantum-based COSMO-RS method has predictive power for a much wider range of compounds, e.g. for gases such as SO2, and qualitative agreement with experiment is observed.
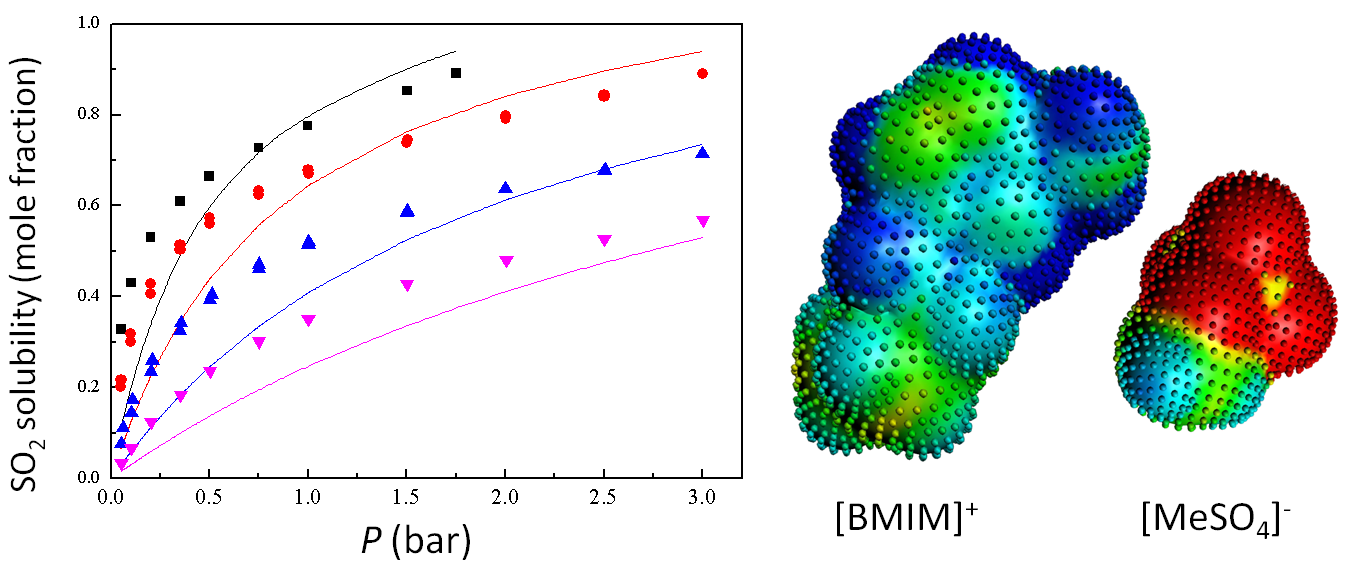
Left: COSMO-RS prediction of SO2 solubilities (solid lines) in [BMIM]+[MeSO4]−, compared with experiments (symbols) at different temperatures (283, 298.1, 323.1, 348.1 K). Right: COSMO surface for BMIM+ and MeSO4–.
With more high-quality experimental data becoming available, the COSMO-RS models could be improved in the future for better quantitative predictions of gas solubilities in ionic liquids.
Prof. Zhigang Lei’s research group (State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, China) has kindly provided the complete ionic liquid database and material for a COSMO-RS tutorial on ionic liquids.
Other papers that may be of interest:
Z. Lei, J. Han, Q. Li, and B. Chen, Process Intensification on the Supercritical Carbon Dioxide Extraction of Low-Concentration Ethanol from Aqueous Solutions, Ind. Eng. Chem. Res., 51, 2730−2737 (2012).
R. Xiong, S. I. Sandler, and R. I. Burnett, An Improvement to COSMO-SAC for Predicting Thermodynamic Properties, Ind. Eng. Chem. Res., 53, 8265–8278 (2014).
Z. Lei, C. Dai, and B. Chen, Gas Solubility in Ionic Liquids, Chem. Rev., 114, 1289−1326 (2014).
Key conceptsCOSMO-RS oil & gas pharma