Gold-Carbon Bonding in Gold-Alkynyl Complexes
Researchers from Brown University and Tsinghua University investigated and analyzed the chemical bonding between gold and carbon in gold-alkynyl complexes combining photoelectron spectroscopy and relativistic quantum chemical calculations. The importance of this work for understanding catalytic mechanisms and reactive intermediates, published in Nature Communications, was also highlighted in Chemical & Engineering News.
The high-level ab initio calculations discovered a rare inverse correlation between bond order and bond strength. “The strong gold–carbon bond underlies the catalytic aptness of gold complexes for the facile formation of terminal alkynyl–gold intermediates and activation of the carbon–carbon triple bond”, the authors said.
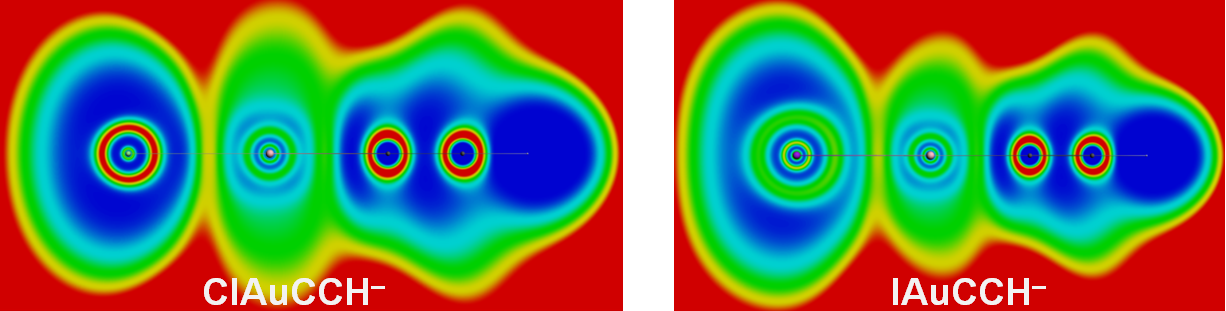
Electron Localization Function (ELF) for gold-alkynyl complexes LAuCCH–, with L=Cl (left) and L=I (right)
Links: Chemical bonding analysis with ADF
H.-T. Liu, X.-G. Xiong, P. D. Dau, Y.-L. Wang, D.-L. Huang, J. Li, and L.-S. Wang, Probing the nature of gold-carbon bonding in gold-alkynyl complexes, Nature Comm. 4, 2201 (2013).
S. K. Ritter, Probing Gold’s Catalytic Prowess, Chem. Eng. News, 91, 24 (2013).
Key conceptsADF bonding analysis catalysis inorganic chemistry Relativistic DFT