Insights into the components of MAO mixtures
Methylaluminoxane (MAO) is one of the most commonly utilized co-catalysts in the single-site production of polyolefins catalyzed by metallocenes. However, its exact structure has eluded experimental characterization because of the dynamic equilibrium between various species comprising the MAO mixture, including oligomers with the general formula (AlOMe)n∙(AlMe3)m as well as the trimethylaluminum dimer (TMA), which is ever-present in solution.
Modeling MAO has proved to be challenging as well: neither standard GGA nor hybrid functionals are able to calculate the correct magnitude or sign for the enthalpy and Gibbs free energy associated with TMA dimerization. Hence, more expensive approaches have been used in the past. This paper shows that dispersion-corrected density functional theory (revPBE+D3) calculations yield reliable thermochemical parameters for TMA dimerization at a reasonable computational cost. Therefore, researchers from Eva Zurek’s group were able to optimize the geometries of many more MAO species than had been considered in prior investigations. The “rescan” keyword available in ADF allowed for the removal of spurious imaginary modes (associated with rotations of methyl groups) from the frequency calculations. Thus, the Gibbs free energies for a plethora of species likely to be present in MAO could be computed reliably and then employed in a Boltzmann distribution to estimate their abundances as a function of temperature.
At room temperature a wide range of species were found to be important components of MAO, and the computed average molecular weight of 1077 g/mol, as well as a C:Al:O ratio of 1.22:1:0.89 matched well with experimental estimates. High temperatures decreased the average molecular weight and shifted the equilibrium towards (AlOMe)n cage structures plus free TMA. (AlOMe)n∙(AlMe3)m oligomers are thought to be precursors to the constituents of MAO active in polymerization, but their abundance is predicted to diminish at higher temperatures. These computations thus provide an explanation for the sudden drop in the polymerization rate at increased temperatures and pave the way to further studies that use this temperature to optimize polymerization conditions.
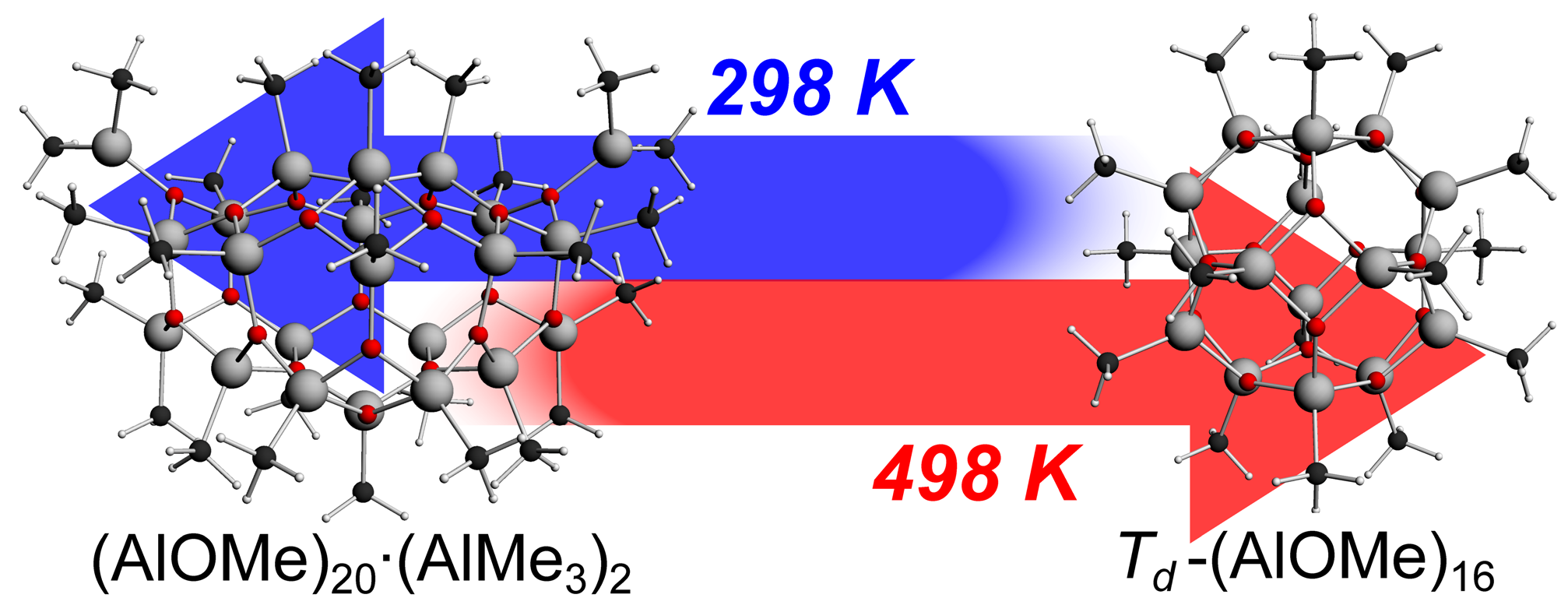
At elevated temperatures the thermodynamic equilbrium for MAO structures shift from trimethylaluminum-bound nanotubes to AlOMe cages, explaining the decreased catalytic activity for polymerization.
Z. Falls, N.Tymińska, and E. Zurek, The Dynamic Equilibrium Between (AlOMe)n Cages and (AlOMe)n·(AlMe3)m Nanotubes in Methylaluminoxane (MAO): A First-Principles Investigation, Macromolecules 47, 8556-9569 (2014)
Key conceptsADF catalysis Dispersion inorganic chemistry oil & gas Reactivity