Insights into the instabilities of halide perovskite with ReaxFF
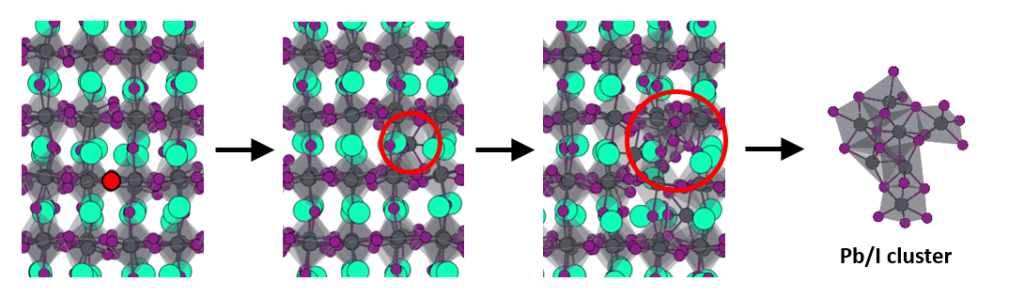
Halide perovskites have attracted enormous attention over the recent years. The combination of their high conversion efficiencies, low production costs, and ease of fabrication make them ideal candidates for use in solar cell technology. Despite these beneficial material properties, the commercialization of perovskite solar cells is hindered by their poor long-term stability, caused by a variety of material intrinsic processes which are not yet fully understood. In a recent paper from researchers from Eindhoven University of Technology (TU/e) and Pennsylvania State University (PSU) shed some light onto the atomistic details of degradation processes in halide perovskites by using reactive molecular dynamics simulations with ReaxFF.
This the first study of a halide perovskite (CsPbI3) with ReaxFF. I/Pb/Cs parameters were obtained from accurate quantum-mechanical reference data and a Monte Carlo optimization. The resulting parameter set is employed to investigate a range of dynamical and reactive processes in the inorganic halide perovskite.
The new ReaxFF parameters were able to predict the proper phase and dynamical behavior of the halide perovskite. The atomistic mechanism by which the perovskite lattice decomposes into PbI2 was uncovered by the reactive molecular dynamics simulations.
M. Pols, J. M. Vicent-Luna, I. Filot, A. C. T. van Duin, and S. Tao, Atomistic Insights Into the Degradation of Inorganic Halide Perovskite CsPbI3: A Reactive Force Field Molecular Dynamics Study, J. Phys. Chem. Lett. 12, 5519–5525 (2021).
Key conceptsheavy elements inorganic chemistry perovskite ReaxFF solar cells