Insights in Mo (di)hydride bonds with ETS-NOCV
Bonding interactions in non-classical hydride/dihydrogen molybdocenes and their interconversion to trihydrides have been analyzed with ETS-NOCV in a recent inside-cover featured article. Ansa-bridges with a small bite angle make the Mo center more sterically available, favoring the non-classical hydride/dihydrogen complex for [(C5H4)2CMe2MoH(H2)]+ complexes. On the other hand, the trihydride configuration is favored for the less accesible Mo in ansa-bridged complexes with a heavier central atom (Si, Ge, Sn, Pb).
In [(C5H4)2CMe2MoH(H2)]+ the trihydride is a low-lying TS for hydrogen exchange. The ETS-NOCV analysis shows that the orbital interactions are much more favorable in the trihydride TS, almost fully counteracting the strongly increased steric repulsion in the TS.
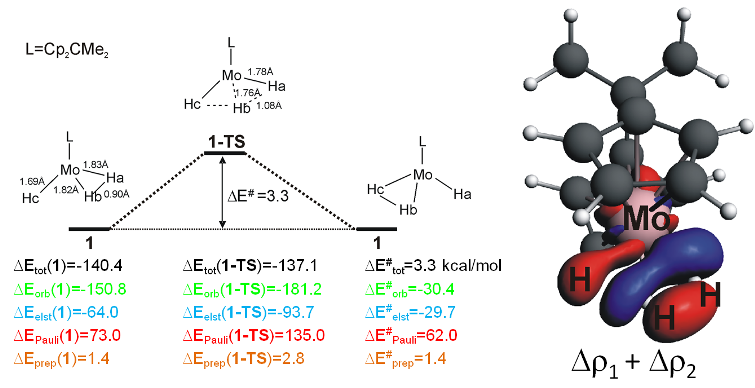
Right: Energy decomposition analysis of Mo trihydride and TS for hydrogen exchange. Left: ETS-NOCV deformation density of the most important interactions in the TS for the hydrogen exchange reaction.
See also: Web presentation on ETS-NOCV by Mariusz Mitoraj.
L. P. Piekos and M. P. Mitoraj, Theoretical description of dihydrogen/hydride and trihydride molybdocene complexes: An insight from static and molecular dynamics simulations , J. Comp. Chem. 34, 294-304 (2013)
See also:M. P. Mitoraj et al., Applications of the ETS-NOCV method in descriptions of chemical reactions, J. Mol. Mod. 17, 2337-2352 (2011)