Is Metallophilicity Really Attractive?
Metallophilicity (M-M’ closed-shell interaction) was discovered in the early 1970s, when close metal-metal contacts were observed extensively in d10-d10 (AuI-AuI, AgI-AgI, CuI-CuI, Pd0-Pd0, Pt0-Pt0) and d8-d8 (RhI-RhI, IrI-IrI, PtII-PtII, PdII-PdII) transition metal complexes. Metallophilicity is usually defined as an ‘attraction’ among closed-shell metal centers, and is widely regarded as a driving force in the fabrication of self-assemblies made by closed-shell d8 and d10 metal complexes. The self-assembled closed-shell metal complexes have wide applications in organic semiconductors, biosensing and OLEDs. The origin of metallophilicity remains controversial, particularly in the role of spd orbital hybridization and relativistic effect. In a recent PNAS article, Wan, Yang, Che and coworkers used ADF to clearly decompose the metallophilic interaction into orbital interaction, electrostatic interaction, dispersion and Pauli repulsion. The calculation results reveal that at close M-M’ distance in the X-ray crystal structures of d8 and d10 organometallic complexes, M-M’ closed-shell interaction is repulsive in nature due to strong M-M’ Pauli repulsion.
By comparing relativistic (ZORA) to non-relativistic basis sets in ADF, it was found that relativistic effects facilitates (n+1)s-nd and (n+1)p-nd orbital hybridization of the metal atom, where (n+1)s-nd hybridization induces strong M-M’ Pauli repulsion and repulsive M-M’ orbital interaction, while (n+1)p-nd hybridization suppresses the M-M’ Pauli repulsion. These calculations rationalize the shorter Ag-Ag’ distances compared to Au-Au’ distances, where weaker Ag-Ag’ Pauli repulsion plays an important role. Although M-M’ interaction is repulsive in nature, the linear coordination geometry of d10 metal complex suppresses the L-L’ (ligand-ligand) Pauli repulsion, while retaining the strength of attractive L-L’ dispersion, leading to a close unsupported M-M’ distance which is shorter than the sum of metal atom’s van der Waals radius (rvdw).
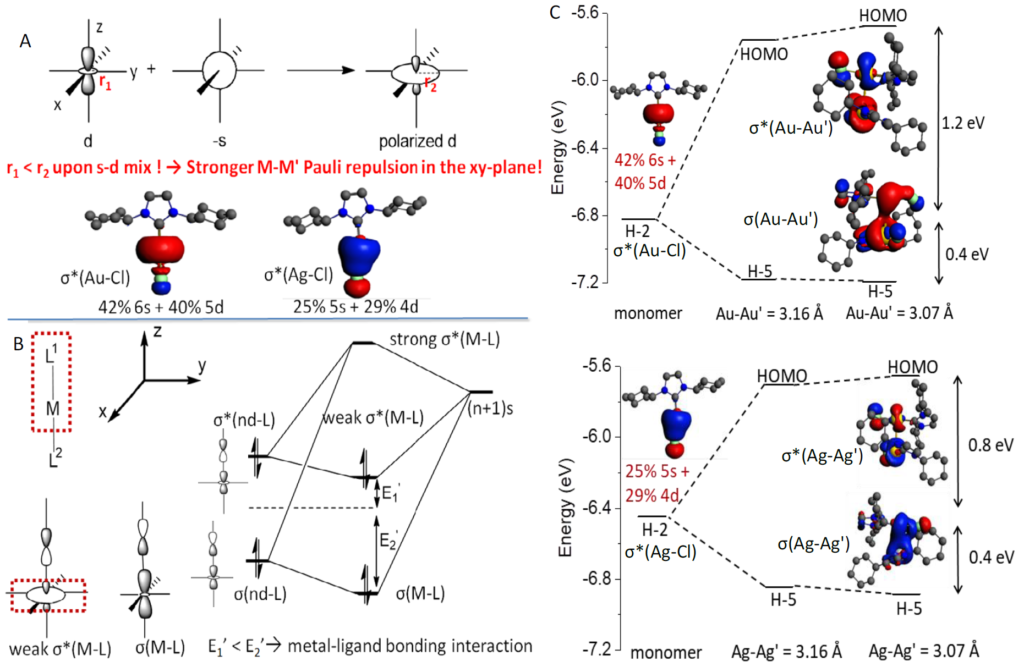
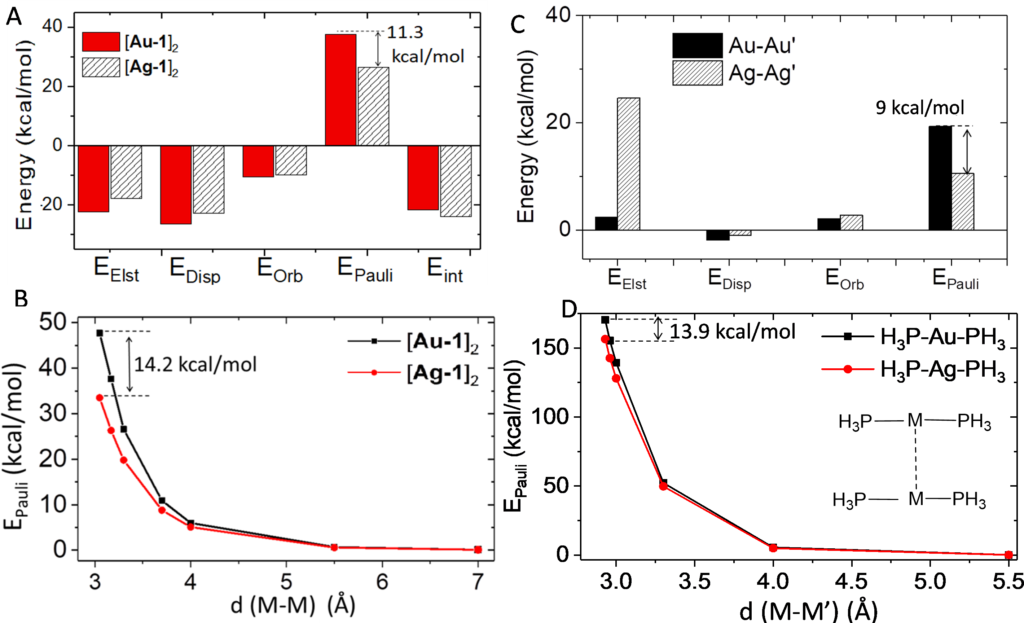
Wan, Q.*; Yang, J*; To, W.-P.; Che, C.-M.* Strong metal–metal Pauli repulsion leads to repulsive metallophilicity in closed-shell d8 and d10 organometallic complexes. PNAS 2020.
Key conceptsADF bonding analysis heavy elements inorganic chemistry OLEDs Relativistic DFT