SiC as anode material for sodium ion batteries – a DFT study
Rechargeable sodium-ion batteries (NIB) are similar in function to the widely used lithium-ion batteries (LIB), however, NIBs are comparatively cheaper, safer and use the more abundant Na+ as charge carrier. Battery performance strongly depends on the anode, and sodium does not intercalate into the standard graphite material used commonly as electrodes in LIBs.
A recent study investigates silicon carbide (SiC) as a potential anode for NIBs. SiC is widely used as a strong high endurance material. Since it is easily producible in many polyforms, SiC could be an attractive anode material for NIBs. Sodium intercalation has been studied in three polytypes (2H-SiC, 4H-SiC and 3C-SiC), both in pure materials and vacancy containing hosts. Periodic density functional theory calculations with BAND show that sodium can intercalate in SiC with vacancies, but not in pure SiC. The intercalation of Na in di-vacancy 2H-SiC is depicted below.
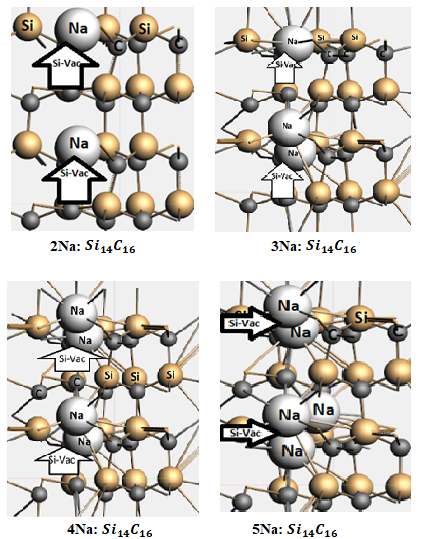
The 2H polytype, which contains the highest amount of hexagonality shows the lowest Na+ diffusion barriers, with optimal diffusion in the z-direction. 2H-SiC also appears to have reasonable anodic electrochemical properties, with a calculated operating voltage of 0.72 V and a specific capacity of 85 mA h g−1.
Majid, A.; Hussain, K.; Khan, S. U.; Khan, S. U., First principles study of SiC as the anode in sodium ion batteries, New J. Chem. 44, 8910–8921 (2020).
Key conceptsBAND batteries materials science