Unraveling f-block bonding interactions: novel uranium complexes
In a series of break-through papers, Prof. Steve Liddle and his collaborators report the synthesis, isolation, and characterization of new uranium complexes.
In a sustained, exciting effort to establish and understand novel chemical bonds in f-block elements, they have discovered for the first time an f-block cyclobutadienyl complex (Nat. Commun. 4, 2323), a terminal U-N triple bond (Science 337, 717, Nat. Chem. 5, 482), O=U(VI)=C(PR2)2 (J. Am. Chem. Soc. 134, 10047), and U(V) carbenes (Angew. Chem. Int. Ed. 50, 2383).
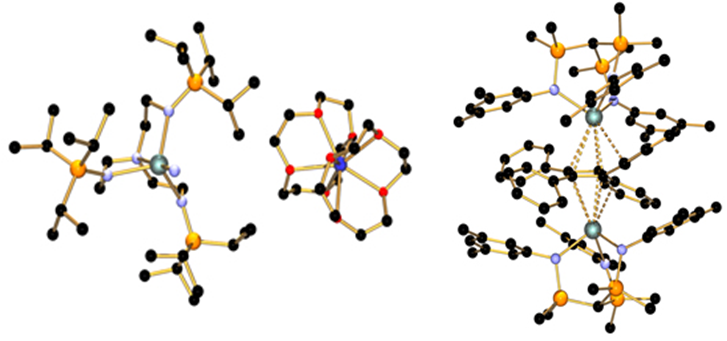
First U(V)-N triple bond (left), later oxidized to the first U(VI)-N triple bond. First U-cyclobutadienyl complex (right).
Relativistic DFT calculations with ADF afforded invaluable insight in the chemical bonds of these latest uranium complexes and how they compare to longer-established U bonds and analogous bonds in d-block complexes. The bonding interactions are routinely studied through bond orders (Mayer, Nalewajski-Mrozek), QTAIM, NBO, orbital populations, and MDC charges and spin densities.
Bonding interactions in uranium complexes
Although the 5f-electrons are predominantly non-bonding, they do participate in U-X multiple bonds as well as carbocyclic π-electron ligands.
Uranium’s oxidation state can strongly affect bond orders as well as the relative participation of 5f and 6d orbitals in the π-bonds. At short U-X distances, the N 2pz orbital has anti-bonding interactions with 5f and 6d orbitals, pushing the σ-bond higher in energy than the π-bonds for the U-N triple bond. Although the U-N distance shrinks from 1.825 to 1.799 Å upon oxidation from U(V) to U(VI), the Mayer bond index is hardly affected (2.91, 2.92).
While there is a strong covalent, δ-backbonding, interaction between the U 5f-orbitals and the central arene ring in inverted sandwich complexes (Nature Chem. 3, 454), the interaction between the two U centers sandwiching cyclobutadienyl are predominantly electrostatic owing to a size mismatch and small orbital overlap.
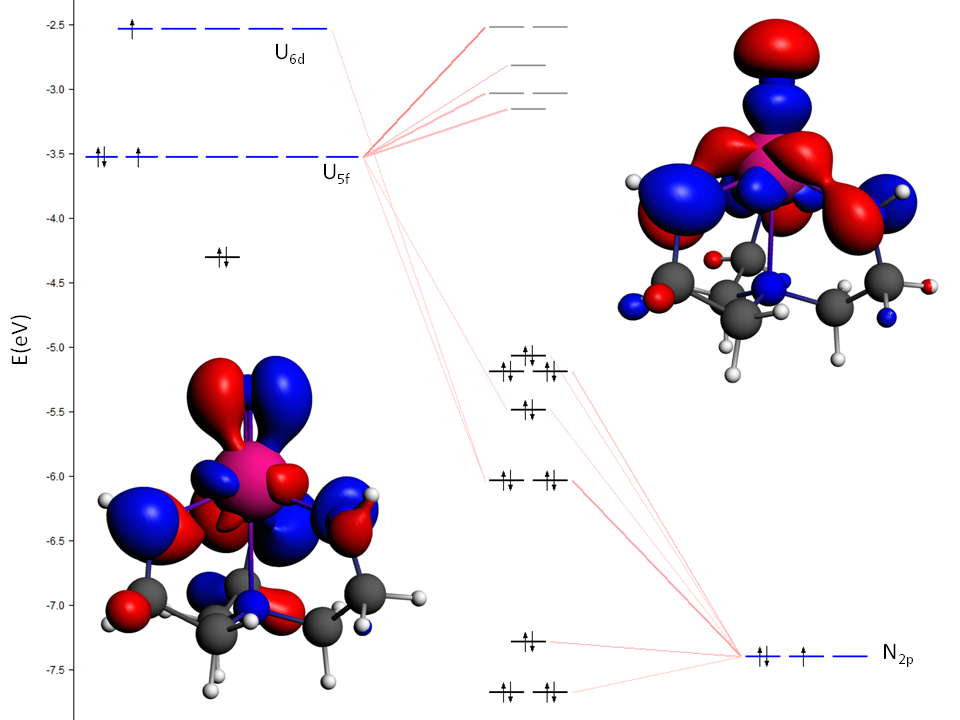
U-N interactions in a model complex with a terminal U(VI)-N triple bond (truncated from experimental [U(N)(TrenTIPS)]; TrenTIPS = {N(CH2CH2NSiiPr3)3}3−, iPr = CH(CH3)2)). The σ-bond (HOMO, top right) is higher in energy than the two quasi-degenerate π-bonds (one shown, bottom left).
The Liddle group also investigates single-molecule magnets, catalytic CO activation, and has synthesized and characterized various other interesting U-complexes. Visit their website to learn more.
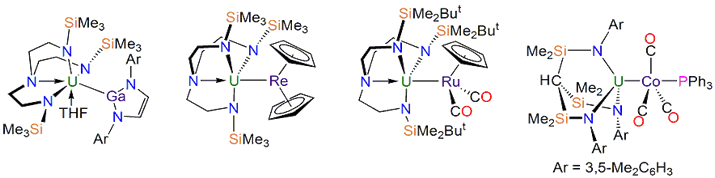
More interesting U bonds established by the Liddle group. Left to right: the first U-Ga bond; the first U-Re bond; the first U-Ru bond; the first U-Co bond.
D. Patel, J. McMaster, W. Lewis, A. J. Blake, and S. T. Liddle, Reductive assembly of cyclobutadienyl and diphosphacyclobutadienyl rings at uranium, Nature Commun. 4, 2323 (2013)
D. M. King, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake, and S. T. Liddle, Isolation and characterisation of a uranium(VI)-nitride triple bond, Nature Chem. 5, 482-488 (2013)
Selected references to recent papers
D. M. King, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake, and S. T. Liddle, Isolation and characterisation of a uranium(VI)-nitride triple bond, Nature Chem. 5, 482-488 (2013)
D. P. Mills, O. J. Cooper, F. Tuna, E. J. L. McInnes, E. S. Davies, J. McMaster, F. Moro, W. Lewis, A. J. Blake, and S. T. Liddle, Synthesis of a Uranium(VI)-Carbene: Reductive Formation of Uranyl(V)-Methanides, Oxidative Preparation of a [R2C═U═O]2+ Analogue of the [O═U═O]2+ Uranyl Ion (R = Ph2PNSiMe3), and Comparison of the Nature of UIV═C, UV═C, and UVI═C Double Bonds, J. Am. Chem. Soc. 134, 10047−10054 (2012)
O. J. Cooper, D. P. Mills, J. McMaster, F. Moro, E. S. Davies, W. Lewis, A. J. Blake, and S. T. Liddle, Uranium-Carbon Multiple Bonding: Facile Access to the Pentavalent Uranium Carbene [U{C(PPh2NSiMe3)2}(Cl)2(I)] and Comparison of UV=C and UIV=C Double Bond, Angew. Chem. Int. Ed. 50, 2383-2386 (2011)
D. P. Mills, F. Moro, J. McMaster, J. van Slageren, W. Lewis, A. J. Blake, and S. T. Liddle, A delocalised arene-bridged diuranium single molecule magnet, Nature Chem. 3, 454-460 (2011)
Key conceptsADF bonding analysis heavy elements Relativistic DFT