Unraveling structure and reactivity of Pt complexes: NMR & EPR
A recent combined experimental and theoretical study by the Kaupp group scrutinizes transition metal phosphanide chemistry which helps to understand catalytic processes involved in C-P bond formation and breaking.
The reaction of a Pt-PPh3 with a Pt(0) complex was monitored with NMR, corroborated by DFT calculations for the structural assignment of the reaction intermediates.
Pt(0) can reversibly insert into the ortho-C-H of the triphenylphosphine, leading to a binuclear μ-hydrido complex. For this meta-stable complex, ADF calculations helped to clearly establish the most likely isomer to match the experiment chemical shifts and couplings. Since this complex is in equilibrium with the reactants, ultimately, the thermodynamically favored phosphanide product is formed through C-P bond scission of one of the phosphorus-phenyl bonds.
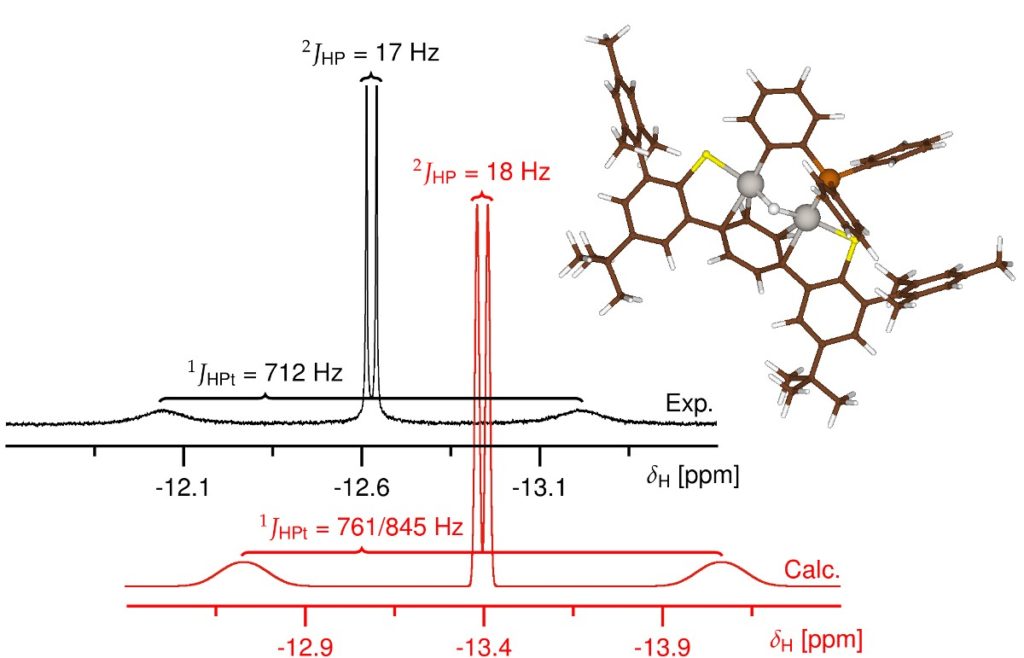
The NMR calculations with ADF, including ZORA and COSMO solvation gave very satisfactory agreements for 1H, 31P, and 195Pt NMR shifts and couplings.
The redox chemistry of the C-P insertion product was also further investigated. EPR measurements revealed that a thiolate rather than metal centred redox chemistry occurs. Also these experimental data were supported though DFT calculations with ADF (g-factor and A-tensor).
Sample input files for NMR and EPR parameter calculations for the two products are available.
Berkefeld, A., Reimann, M., Hörner, G., Kaupp, M., Schubert, H.. C–P vs C–H Bond Cleavage of Triphenylphosphine at Platinum(0): Mechanism of Formation, Reactivity, Redox Chemistry, and NMR Chemical Shift Calculations of a μ-Phosphanido Diplatinum(II) Platform. Organometallics 39, 443-452 (2020).
Key conceptsADF catalysis EPR NMR Reactivity Relativistic DFT spectroscopy