Zeolite-catalyzed hydrolysis: DFT/DFTB calculations
The catalytic hydrolysis of propylene oxide in ZSM-5 was investigated using multi-layered ADF/DFTB calculations with QUILD. Using dispersion-corrected DFT (BP86-D3) at the high level and third-order DFTB (DFTB3) at the low level, both monopropylene glycol and dipropylene glycol are preferentially formed via a concerted mechanism, with a lower barrier for the formation of monopropylene glycol. Selectivity could be further increased by reducing the pore size to disfavor dipropylene glycol formation.
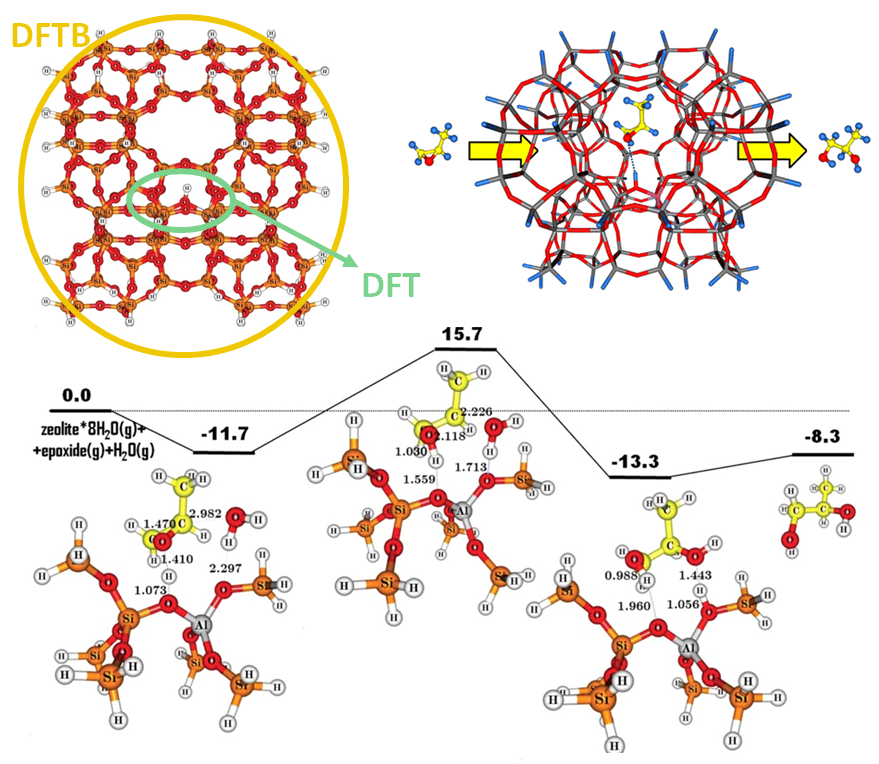
Multi-layer DFT/DFTB set-up and product flow (top) and concerted reaction pathway in ZSM-5 for monopropylene glycol formation
Y. Horbatenko, J. P. Pérez, P. Hernández, M. Swart, and M. Solà, Reaction Mechanisms for the Formation of Mono- And Dipropylene Glycol from the Propylene Oxide Hydrolysis over ZSM-5 Zeolite, J. Phys. Chem. C, 118, 21952–21962 (2014)
Key conceptsADF catalysis DFTB Dispersion multi-layer oil & gas Reactivity