5f covalency in Americium hexachloride
The Cl K-edge XAS of Americium(III) Hexachloride were simulated and analyzed based on spin-orbit DFT transition dipole moment method. Direct evidence of 5f and 3p orbital mixing in Am–Cl bond was observed. Combining experimental Cl K-edge XAS and theoretical simulation, the comparison between AmCl63- and isoelectronic EuCl63- confirms Seaborg’s 1954 hypothesis that AmIII 5f-orbital covalency was more substantial than 4f-orbital mixing for EuIII.
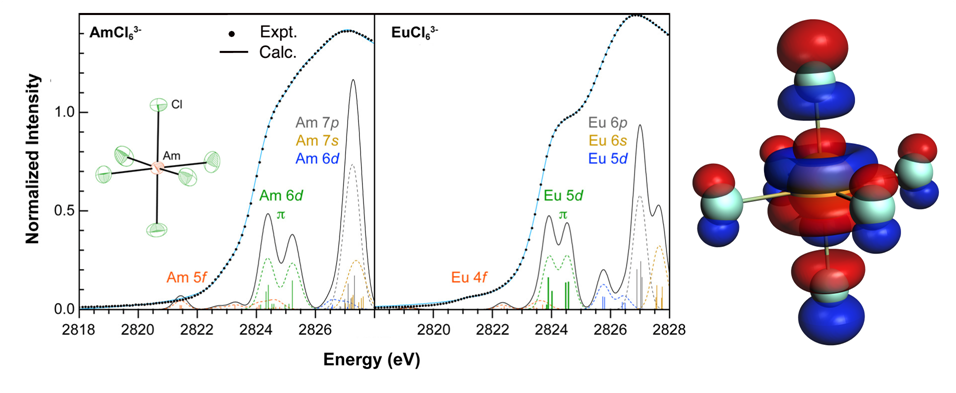
Left: The experimental data (•) and the curve fitted model (blue trace) for the Cl K-edge XAS spectrum from (PPh4)3AmCl6 and (PPh4)3EuCl6. Comparison between the experimental Cl K-edge XAS spectrum (•) and orbital-difference DFT calculations (black trace). The orange, green, purple, and grey bars and dashed traces represent the energy and oscillator strength for the calculated transitions involving 5f- and 6d-final states. Right: t1u orbital in AmCl63- (HOMO) showing involvement of both Cl and Am 5f-orbitals.
J. N. Cross, J. Su, E, R. Batista, S. K. Cary, W. J. Evans, S. A. Kozimor, V. Mocko, B. L. Scott, B. W. Stein, C. J. Windorff, P. Yang, Covalency in Americium(III) Hexachloride, J. Am. Chem. Soc. 139, 8667–8677 (2017).
Key conceptsADF bonding analysis heavy elements NEXAFS Relativistic DFT XAS