Spin Orbit Coupling effect on spin-forbidden reaction mechanisms
A spin forbidden reaction occurs with a change of the total electronic spin from reactants to products. The mechanistic study is therefore not trivial, since the reaction does not occur on a single adiabatic potential energy surface (PES), but rather on two spin diabatic PESs crossing at the so called “Minimum Energy Crossing Point” (MECP), which is available in ADF2016. In this work the mechanism of the reaction of molecular oxygen with a gold hydride complex was analyzed, involving the change of the total electronic spin from triplet to singlet. This work introduces for the first time the Spin Orbit Coupling effects (SOC, using ZORA Hamiltonian). In the SOC approach, the reaction occurs on a single adiabatic PES where a Transition State (TS) can be calculated.
In the figure below the MECP and SOC approaches for studying spin-forbidden reaction mechanisms are compared (left), and the resulting reaction profiles for the oxidative addition path. For a heavy atom like gold, we found a sizeable SOC effect of 3.2 kcal mol-1 in lowering the activation barrier, with respect to MECP. Importantly, with the inclusion of SOC effects, a transition state can be computed, thus allowing the calculation of activation free energies, which is not possible using the MECP approach, since the MECP is not a stationary point.
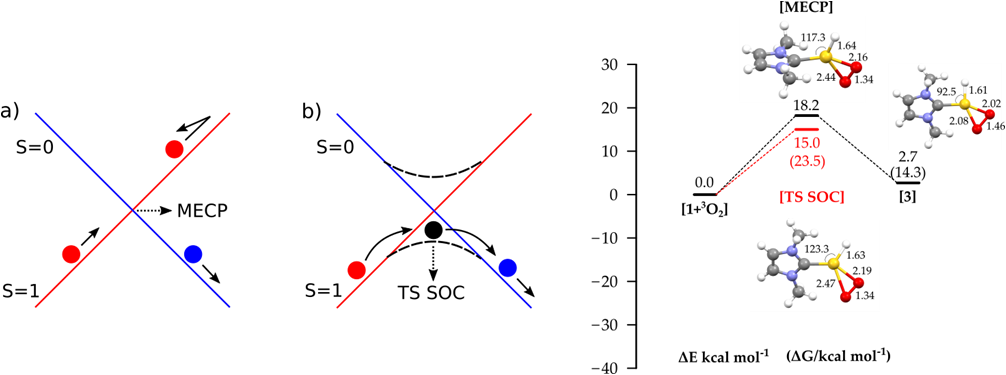
Comparison of a) scalar relativistic and b) SOC approaches (left); reaction profile for the O2 oxidative addition into the NHCAu-H bond (right)
C. A. Gaggioli, L. Belpassi, F. Tarantelli, D. Zuccaccia, J. N. Harvey, and P. Belanzoni, Dioxygen insertion into the gold(I)–hydride bond: spin orbit coupling effects in the spotlight for oxidative addition, Chem. Sci. 7, 7034-7039 (2016).
Key conceptsADF catalysis heavy elements inorganic chemistry Reactivity Relativistic DFT